Abstract
Forensic entomology investigates postmortem interval (PMI) estimation using insect evidence. We estimated the minimum PMI of a putrefied male cadaver using pupae in the soil and maggots found in the body. Most of the maggots, collected during the autopsy, were postfeeding third instar larvae with empty crop contents, which developed freezing injuries when the cadaver was placed in the freezer. Pupae in the soil were collected 45 days after the discovery of the body. DNA barcoding revealed that most pupae and maggots were Chrysomya pinguis, with a few exceptions. The minimum PMI was estimated at 10 days before the discovery time based on the scene investigation, maggot developmental stage, distribution of pupae moving away from the body toward pupariation sites, DNA barcoding results, and weather information. To reduce the gap between the minimum and maximum PMI values, complete entomological evidence collection should be conducted at the time of discovery.
Go to : 
REFERENCES
1. Kang CS, Park SO, Kim JI, et al. General entomology. 2nd ed.Seoul: Jungmoongak;2000. p. 233–40.
2. Villet MH, Richards CS, Midgley JM. Contemporary precision, bias and accuracy of minimum postmortem intervals estimated using development of carrion-feeding insects. Amendt J, Goff LM, Campobasso CP, editors. ., eds.Current concepts in forensic entomology. Dordrecht: Springer;2010. p. 109–37.

3. Hall M, Whitaker A, Richards C. Forensic entomology. Marquez-Grant N, Roberts J, editors. Forensic ecology handbook from crime scene to court. Hoboken, NJ: Wiley-Blackwell;2012. p. 111–40.

4. Lee RE Jr. A primer on insect cold-tolerance. Denlinger DL, Lee RE, editors. Low temperature biology of insects. Cambridge: Cambridge University Press;2010. p. 3–34.

5. Gennard D. Forensic entomology: an introduction. 2nd ed.Hoboken, NJ: Wiley-Blackwell;2012. p. 127–8.
6. Yang ST, Shiao SF. Temperature adaptation in Chrysomya megacephala and Chrysomya pinguis, two blow fly species of forensic significance. Entomol Exp Appl. 2014; 152:100–7.
7. Cui H, Min J. Forensic entomology. Chongqing (CH): Chongqing Publishing House;2000. 155.
8. Anderson GS. Minimum and maximum development rates of some forensically important Calliphoridae (Diptera). J Forensic Sci. 2000; 45:824–32.

Go to : 
 | Fig. 1.After pupae samples were collected from the scene, flags were placed at the discovery site. The ellipse with a dotted line indicates the area where the body was located. The arrow indicates the estimated trace of postfeeding larvae to find places for pupation. |
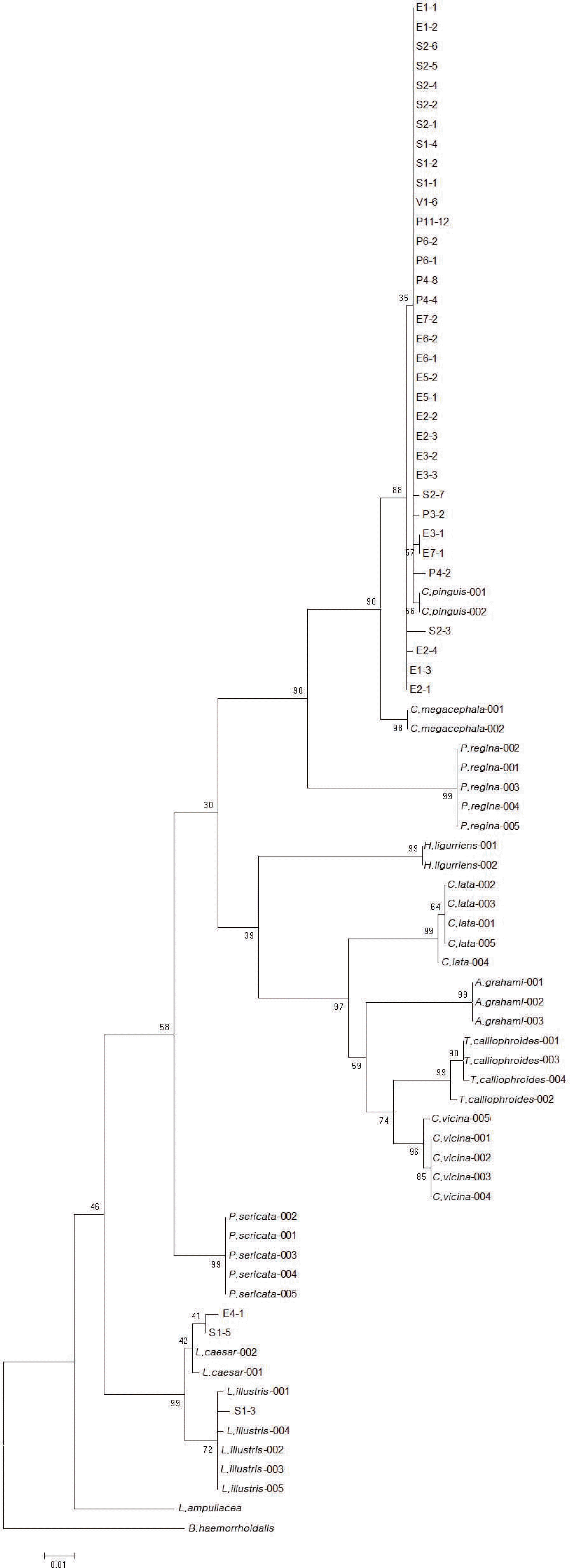 | Fig. 2.DNA barcoding result for 30 maggots and 7 pupae revealed that all except for 3 maggots were Chrysomya pinguis. Two and one maggots were Lucilia caesar and Lucilia illustris, respectively. The initials E, V, S, and P indicate maggots from the body, from the plastic cover for the body, from the shroud, and pupae from the scene, respectively. C, pinguis, Chrysomya pinguis; C. megacephala, Chrysomya megacephala; P. regina, Phormia regina; H. ligurriens, Hemipyrellia ligurriens; C. lata, Calliphora lata; A. grahami, Aldrichina grahami; T. calliophroides, Triceratopyga calliphroides; C. vicina, Calliphora vicina; P. sericata, Phaenicia sericata; L. caesar, Lucilia caesar; L. illustris, Lucilia illustris; L. ampullacea, Lucilia ampullacea; B. haemorrhoidalls, Boettcherisca haemorrhoidalls.
|
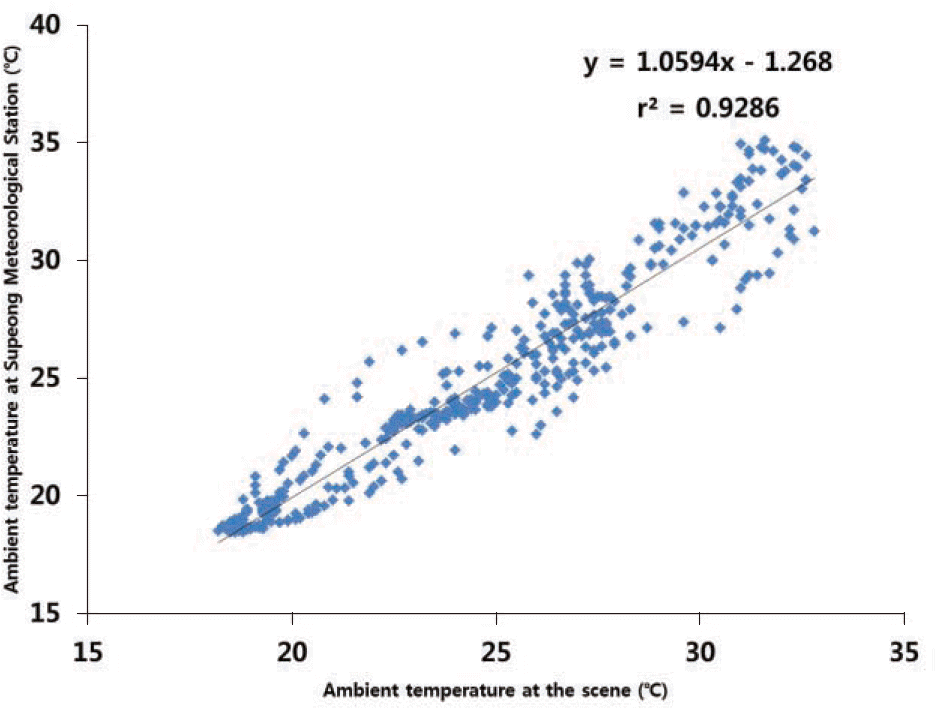 | Fig. 3.A linear regression curve of ambient temperatures from the scene and the nearest meteorological station shows a close correlation between two sites. |
Table 1.
A spread sheet to calculate the accumulated degree hours (ADH) for Chrysomya pinguis larvae (base temperature: 12.3∗ C)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download