Abstract
The diffuse midline glioma H3 K27M-mutant was only added recently to the World Health Organization (WHO) Classification of Tumours of the Central Nervous System. While similar tumors are found in the midline, this particular mutation represents the majority of diffuse gliomas in the brainstem. Classified by WHO as grade IV tumors, these are aggressive and bear a poor prognosis for the patient. This report describes a case involving a 37-year-old woman with a histologically confirmed diffuse midline glioma H3 K27M-mutant in the bilateral thalamus and midbrain. The following discussion describes typical characteristics observed with computed tomography and magnetic resonance imaging. Given the rarity of diffuse intrinsic pontine gliomas in adults and the general lack of studies investigating this poorly understood entity, we report critical findings for contribution to the existing scarce literature on the topic.
The diffuse midline glioma H3 K27M-mutant is a distinct subtype of the infiltrative tumor, which represents a majority of diffuse brainstem gliomas. Recently, this subtype was recognized as a new diagnostic entity in the forthcoming edition of the World Health Organization (WHO) Classification of Tumours of the Central Nervous System (1). Typically, diffuse midline gliomas H3 K27M-mutant cases occur in the pons and thalamic regions (2). Herein, we report a case involving a 37-year-old woman with a histologically confirmed diffuse midline glioma H3 K27M-mutant in the bilateral thalamus and midbrain. The following discussion describes the typically observed computed tomography (CT) and magnetic resonance imaging (MRI) characteristics of this entity. We also performed a review of the relevant literature of this aggressive disease.
A 37-year-old woman presented with dizziness. This symptom gradually progressed for one year without any associated neurological symptoms. General physical and neurological examinations did not reveal any other abnormalities. Further, laboratory investigations, including examination of the cerebrospinal fluid, yielded negative results.
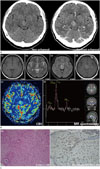
Contrast-enhanced brain CT revealed an ill-defined, hyperattenuated lesion, with subtle enhancement in the bilateral thalamus, measuring 1.7 × 2.0 × 2.1 cm (Fig. 1A). The MRI revealed a diffuse infiltrative lesion located in the bilateral thalamus and midbrain. There was a mild mass effect observed with iso-signal intensity in T1-weighted images, and high signal intensity in T2-weighted images and fluid-attenuated inversion recovery images. This lesion exhibited heterogeneous enhancement in the right thalamus, following injection of gadolinium (Fig. 1B). Proton magnetic resonance spectroscopy images of the center of the mass were also obtained. An increased choline-to-creatine ratio and a decreased concentration of N-acetylaspartate were observed. Additionally, perfusion MRI revealed elevated cerebral blood volume and cerebral blood flow at the site of enhancement.
Stereotactic biopsy was subsequently performed. The lesion exhibited proliferation of well-differentiated fibrillary astrocytes, demonstrating moderately increased cellularity and occasional nuclear atypia, consistent with diffuse astrocytoma. Immunohistochemistry was performed using a polyclonal antibody recognizing histone H3.3 and H3.1 tail epitopes, which identified the lesion as histone H3 K27M-mutant protein (Fig. 1C).
The WHO Classification of Tumours of the Central Nervous System uses an integrated diagnostic scheme, incorporating both morphological and molecular features. Emerging evidence indicates that diffuse brainstem gliomas arise in the thalamus, pons and spinal cords of children and young adults. These gliomas are associated with poor prognosis and are categorized as WHO grade IV tumors, regardless of histological features (2).
One narrowly defined group of tumors that primarily occur in children (and infrequently in adults), is characterized by the H3 K27M-mutation encoded by the histone H3 gene H3F3A. These tumors exhibit a diffuse growth pattern and are generally found somewhere around the midline. Less commonly, mutations in the related HIST1H3B gene has also been reported. A newly defined entity, termed “diffuse midline glioma H3 K27M-mutant,” includes tumors previously referred to as diffuse intrinsic pontine gliomas. Identification of this phenotypically and molecularly defined set of tumors can now provide a rationale for therapies directed against the these specific mutations (2).
Retrospective analyses of 47 cases of diffuse midline gliomas positive for histone H3 K27M-mutant by Solomon et al. (2) indicates that the age of patients at the time of diagnosis range from 2 to 65 years of age (median age of 14 years). However, patients with pontine gliomas tend to be younger (median age 7 years) than those with thalamic (median age 24 years) or spinal (median age 25 years) gliomas. Most of the tumors in these studies were located in the pons (36%), followed by the thalamus (32%), spinal cord (19%), third ventricle (6%), hypothalamus (2%), cerebellum (2%), pineal region (2%), and lastly the midbrain (0%) and medulla (0%). Additionally, Aboian et al. (3) reviewed 24 specific cases of diffuse midline gliomas with histone H3 K27M-mutant in pediatric patients, where most of the tumors were found to be in the pons (46%), followed by the thalamus (25%), vermis/fourth ventricle (17%), cervical spine (8%), subcallosal (4%), with none in the midbrain (0%). Our particular case presented with a very rare midbrain location of the histone H3 K27M-mutant.
The clinical features of the H3 K27M-mutation vary depending on the tumor location, where symptoms may include the following: headache, cranial nerve palsy, sensory disturbance, visual disturbance, gait disturbance, motor weakness, personality changes, confusion, memory loss, apathy, emotional lability, and dementia (45). The diffuse midline glioma H3 K27M-mutant is found in critical midline structures such as the spinal cord and brainstem, which preclude surgical resection in most cases. However, it is unclear whether the poor prognosis stemming from these tumors is due to the burden of the tumor, given the exceeding difficulty of resection, or whether it is a direct manifestation of the H3 K27M-mutation. Although the H3 K27M-mutation appears to be found more commonly in diffuse midline gliomas, the mutation also appears to be found infrequently in diffuse gliomas arising peripherally in the cerebral hemisphere. Given that the hallmarks of diffuse non-midline gliomas with H3 K27M-mutation remain undefined and unclear at present, we believe these tumors should not be specified as WHO grade IV (6).
CT findings of brainstem gliomas typically reveal a hypodense or isodense mass (7). The MRI findings usually demonstrate T2 hyperintensity and a heterogeneously enhancing infiltrative mass with T1 hypointensity (7). Importantly, imaging features vary according to tumor type and location. Guillamo et al. (4) have reported four patterns detected in adult brainstem gliomas, identified by MRI: patterns representing non-enhancing diffusely infiltrative tumors; contrast-enhancing localized masses; isolated tectal tumors; and others (posterior exophytic, diffusely infiltrative with enhanced nodule). Forty-six percent of tumors exhibited contrast enhancement that was associated with shorter survival. Presumed “necrosis” on MRI (defined as a zone of irregularly shaped T1 hypointense signal surrounded by contrast enhancement) was found in 20% of cases and strongly correlated with shorter survival. In another study, contrast enhancement was also an unfavorable factor, particularly when the area of enhancement surrounded a low-signal site suggestive of necrosis. In children, however, the prognostic value of contrast enhancement remains controversial (8). Aboian et al. (3) retrospectively reviewed imaging features of pediatric patients with midline gliomas with or without the histone H3 K27M-mutation. They found that diffuse midline gliomas with H3 K27M-mutant exhibited variable imaging features, with thalamic gliomas showing contrast enhancement and necrosis in 50% of patients, pontine gliomas exhibiting contrast enhancement to a variable degree in 67%, and cervical spine gliomas being uniformly enhancing. When they compared diffuse midline gliomas according to the presence or absence of histone H3 K27M-mutation, there was no significant correlation between border characteristics or enhancement, or presence of edema or infiltrative appearance.
Radiological differential diagnosis in typical cases of diffuse brainstem gliomas may indicate conditions such as brainstem encephalitis, demyelinating disease (e.g., multiple sclerosis or acute disseminated encephalomyelitis), neurofibromatosis type 1, and osmotic demyelination. All of these conditions can present with solitary or multifocal, poorly delineated hyperintensity on T2/fluid-attenuated inversion recovery MRI (9). In our case, the lesion involved the bilateral thalamus and midbrain. Lesions involving the bilateral thalamus and midbrain include lymphoma, basilar artery occlusion or artery of Percheron infarction, deep vein thrombosis, flavivirus encephalitis, and Creutzfeldt-Jakob disease (810). Although imaging may suggest tumor grade on the basis of contrast enhancement, definitive grading requires histopathological examination of the excised tissue. Accordingly, stereotactic biopsy is the preferred mode for definitive diagnosis of diffuse midline glioma. Although image-guided stereotactic biopsy of the brainstem is considered to be a safe and reliable procedure, the optimal methods and routes of biopsy remain debatable (10).
Here, we present a rare case of diffuse midline glioma, H3 K27M-mutant that extended to the bilateral thalamus and midbrain. The radiological findings of diffuse midline glioma, H3 K27M-mutant are commonly observed in the pons and thalamic regions; however, in our case, it led to an even more challenging diagnosis in clinical practice. Although it is often difficult to differentiate diffuse midline glioma, H3 K27M-mutant from other similar diseases, it should be considered in the radiological differential diagnosis in patients with bilateral, brainstem lesions.
Figures and Tables
Fig. 1
Diffuse midline glioma, H3 K27M-mutant in the bilateral thalamus and midbrain in a 37-year-old woman, presenting with dizziness.
A. Axial non-enhanced computed tomography scan revealing an ill-defined hyper-attenuated mass in the right thalamus.
B. (a) Axial pre-contrast T1WI revealing a homogeneously hypointense lesion in the right thalamus. (b) Axial T2WI demonstrating a hyperintense lesion in the bilateral thalamus. (c) Axial FLAIR image revealing a hyperintense lesion in the bilateral thalamus. (d) Axial post-contrast T1WI revealing a focal irregular enhancement in the right thalamus. (e) CBV parameter map revealing an increased signal in the right thalamus. (f) MR spectroscopy: an increased choline peak and a markedly decreased N-acetylaspartate peak are apparent in the right thalamus.
CBV = cerebral blood volume, FLAIR = fluid-attenuated inversion recovery, MR = magnetic resonance, T1WI = T1-weighted image, T2WI = T2-weighted image
C. Photomicrograph: (a) hematoxylin and eosin stain, demonstrating dense cellularity and occasional nuclear atypia. (b) Immunohistochemistry for histone H3 K27M-mutant protein revealing the nuclei of a majority of tumor cells (brown stain) with only few normal, residual cells (blue) (original magnification × 200).
References
1. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131:803–820.
2. Solomon DA, Wood MD, Tihan T, Bollen AW, Gupta N, Phillips JJ, et al. Diffuse midline gliomas with histone H3-K27M mutation: a series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol. 2016; 26:569–580.
3. Aboian MS, Solomon DA, Felton E, Mabray MC, Villanueva-Meyer JE, Mueller S, et al. Imaging characteristics of pediatric diffuse midline gliomas with histone H3 K27M mutation. AJNR Am J Neuroradiol. 2017; 38:795–800.
4. Guillamo JS, Monjour A, Taillandier L, Devaux B, Varlet P, Haie-Meder C, et al. Brainstem gliomas in adults: prognostic factors and classification. Brain. 2001; 124(Pt 12):2528–2539.
5. Balasa A, Balasa R, Egyed-Zsigmond I, Chinezu R. Bilateral thalamic glioma: case report and review of the literature. Turk Neurosurg. 2016; 26:321–324.
6. López G, Oberheim Bush NA, Berger MS, Perry A, Solomon DA. Diffuse non-midline glioma with H3F3A K27M mutation: a prognostic and treatment dilemma. Acta Neuropathol Commun. 2017; 5:38.
7. Lakhan SE, Harle L. Difficult diagnosis of brainstem glioblastoma multiforme in a woman: a case report and review of the literature. J Med Case Rep. 2009; 3:87.
8. Fischbein NJ, Prados MD, Wara W, Russo C, Edwards MS, Barkovich AJ. Radiologic classification of brain stem tumors: correlation of magnetic resonance imaging appearance with clinical outcome. Pediatr Neurosurg. 1996; 24:9–23.
9. Osborn AG. Astrocytomas. In : Osborn AG, editor. Osborn's brain: imaging, pathology, and anatomy. 1st ed. Salt Lake City, UT: Amirsys;2012. p. 453–492.
10. Rachinger W, Grau S, Holtmannspötter M, Herms J, Tonn JC, Kreth FW. Serial stereotactic biopsy of brainstem lesions in adults improves diagnostic accuracy compared with MRI only. J Neurol Neurosurg Psychiatry. 2009; 80:1134–1139.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download