Abstract
Localized aortic dissection at the sinus of Valsalva is a rare finding in patients with Stanford type A dissections. This condition can be fatal and difficult to diagnose. In this study, we report a case of a 69-year-old man with aortic dissection at the sinus of Valsalva, which was accurately diagnosed with multiplanar reformatted images of coronary computed tomography angiography, and later confirmed at surgery.
Localized Stanford type A dissection at the sinus of Valsalva is a condition with difficult early diagnosis, and requires emergency surgical treatment (1). Coronary computed tomography angiography (CCTA) with electrocardiography (ECG) gating can be a useful imaging modality for diagnosis of aortic dissection (2). Herein, we report a case of aortic dissection limited to the sinus of Valsalva, focusing on CCTA findings.
A 69-year-old man was transferred to our hospital because of bilateral acute pyelonephritis detected by abdominal CT scan. The patient had complaints of fever, chilliness, and bilateral flank pain for 7 days prior to visiting the local clinic, and did not have any specific hospital history. Methicillin-sensitive Staphylococcus aureus was isolated in blood cultures at the time of admission at the local clinic. Based on suspicion of acute pyelonephritis and bacteremia, the patient was medicated with ceftriaxone and nafcillin for 5 days, however symptoms persisted. At transfer, his vital signs were as follows: blood pressure 100/60 mm Hg, pulse 86/min, respiratory rate 20/min, and body temperature 36.4℃. Five days after transfer, vital signs were still unstable: blood pressure 96/44 mm Hg, pulse 92/min, respiratory rate 17/min, and body temperature 38.0℃. Because a grade III systolic murmur, splinter hemorrhage, and petechiae were present in the physical examination, the attending physician suspected infective endocarditis.
Chest radiography revealed cardiomegaly and blunting of both costophrenic angles, indicating bilateral pleural effusion (Fig. 1A). Cardiac enzymes, such as creatine kinase MB fraction and hs troponin T were increased, with values of 35.02 ng/mL (normal range: 0–6.22 ng/mL) and 0.161 ng/mL (normal range: 0.000–0.013 ng/mL), respectively. Other laboratory tests indicated inflammation: white blood cell 13.650/µL, neutrophils 83%, erythrocyte sedimentation rate 59 mm/h (normal range: 0–20 mm/h), C-reactive protein 29.3 mg/dL (normal range: 0.0–0.3 mg/dL), procalcitonin 67.9 ng/mL (normal range: 0–0.5 ng/mL). ECG revealed wide Q waves in leads II, III, and aVF without ST segment elevation, indicating a chronic inferior wall infarction. Portable echocardiography was performed, and demonstrated decreased wall motion with less contractility in the inferior wall of left ventricle (LV). Concerned with the possibility of ischemic heart disease, CCTA was performed. A 320 slice CT scanner (Aquilion ONE, Toshiba Medical Systems, Nasu, Japan) with three-dimensional volumetric cardiac imaging during the diastole of R-R interval (Gantry rotation time of 350 msec, scan time of 1.224 sec, 0.5 mm slice thickness with 0.25 reconstruction interval) was done and short axis view, 2 chamber view, 4 chamber view, and volume rendering were acquired. In CCTA, 80 mL of contrast agent, Tomoray 320 (Ioversol; Dongguk Pharm, Seoul, Korea), followed by 40 mL of a 50%/50% saline/contrast medium mixture was infused at 4.5 mL/sec by a dual injection system (Stellant, Medrad Inc., Indianola, PA, USA). CT showed suspicious aortic regurgitation displaying an enlarged LV with poor coaptation of the right and non coronary cusps in diastole, as well as mild dilatation of the noncoronary sinus of Valsalva. There was 50% luminal narrowing at the ramus intermedius. We could not find abnormal valve thickening or vegetation, but small aneurysmal dilatation at the non-coronary Valsalva sinus was discovered (Fig. 1B). Trans-esophageal echocardiogram (TEE) could not be performed immediately due to patient's non-cooperation. Therefore, the clinician decided to start conservative therapy on suspicion of ischemic heart disease. Nineteen days later, the first TEE was performed and echocardiography demonstrated the presence of infective endocarditis with severe aortic regurgitation and dilatation of the ascending aorta, vegetations, and decreased contractility in the anteroseptal wall of the LV (Fig. 1C). Follow-up CCTA following TEE showed suspicious infective endocarditis with valvular vegetations, focal calcification, and a dissection flap involving the non-coronary sinus of Valsalva. The dissection flap only involved the sinus of Valsalva (Fig. 1D).
The patient was submitted to surgery 6 days later due to delayed patient's consent. During the procedure, it was found that the annulus of the aortic valve was destroyed by infective endocarditis and there was an aortic dissection present from the non-coronary cusp to the left coronary sinus. The reconstructed image corresponding to the intra-operative picture revealed a dissection flap between the non-coronary cusp and the left coronary sinus (Fig. 1E, F). It was decided to obliterate the dissection site and insert a grafted aorta with a prosthetic aortic valve. Histologic evaluation of the aortic valve showed acute inflammation with fibrinoid necrosis, focal abscess formation, and dystrophic calcification. After surgery, the patient's symptoms vanished, and he was discharged on the 63rd day.
Aortic dissection can occur when the intimomedial layer of the aortic wall is torn by trauma, iatrogenic manipulation, or inflammation. Dissection of aortic wall layers can extend to the proximal and distal aortic parts. The Stanford classification system classifies aortic dissection into two types based on its location. All aortic dissections involving the ascending aorta are defined as “type A” and the rest as “type B.” Type A aortic dissection requires urgent surgical treatment, with dissections limited at the sinus of Valsalva being treated by coronary stenting. Patients with type A aortic dissection have a high risk of aortic wall rupture, causing secondary cardiac tamponade, aortic regurgitation, and visceral malperfusion (3).
Unlike other common type A dissections, detecting localized dissection flaps at the sinus of Valsalva require more attention (1). Radiologists should try to detect these small flaps, as pulsating artifacts often occur at the same site, and small dissection flaps mimic normal coronary cusps (1). Therefore, in this case, multiplanar reformatted (MPR) images (Fig. 1D, E) of the aortic valve were used to diagnose aortic dissection. Particularly, these reformatted images assist in understanding three-dimensional relationships between normal anatomy and pathologic lesions.
Conventional aortography, transesophageal echocardiography, CT, or magnetic resonance imaging can be used to diagnose aortic dissection (4). Many studies have been performed concerning diagnostic accuracy of aortic dissection by echocardiography; recently developed three-dimensional echocardiography provides more helpful information to understand the anatomical structure of dissection than two-dimensional echocardiography (5). However, several limitations exist: the need of a well-trained user, limited availability in hospitals, obscured view of distal ascending aorta and proximal arch trapping air in the trachea and left main bronchus, and an obscured dissection flap under severe aortic valve calcification (6). Furthermore, detected lesions by echocardiography are difficult to differentiate between vegetation, calcification and tumor (7). The selection of initial diagnostic tools for suspected aortic dissection is controversial (48). MPR images of CCTA, which show very high sensitivity, specificity and contains various reconstruction techniques, can also be used as a valuable method to detect aortic dissection (9).
In this case, aortic dissection at the sinus of Valsalva with valvular vegetations and focal valvular calcification was confirmed by follow-up CCTA. The abscess of the Valsalva sinus was suspected to be the origin site of the dissection. Although mixed localized dissection and abscess were not differentiated in CCTA, MPR images helped to understand lesion extent and direction, as well as evaluate other combined lesions. After being diagnosed with aortic dissection at the sinus of Valsalva by CCTA, the patient was submitted to emergency surgery and recovered completely.
Although type A aortic dissection is a well-known pathology, aortic dissection which is limited at the sinus of Valsalva needs more attention for detection and can be easily misdiagnosed (127). CCTA with multiplanar reformation may be a useful reconstruction technique for localized aortic dissection diagnosis.
Figures and Tables
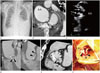
Fig. 1
Localized aortic dissection at the sinus of Valsalva with vegetations and dystrophic calcification in a 69-year-old man.
A. Chest radiograph shows cardiomegaly, pulmonary congestion, and bilateral pleural effusion.
B. Short axis view of initial CCTA represents dilated AA without evidence of dissection. There is a small contrast filled outpouching which communicates with the non-coronary Valsalva sinus (arrow).
C. Trans-esophageal echocardiogram demonstrates dilatation of AA and vegetations of aortic valve (arrows).
D. Coronal oblique MPR image in the follow-up CCTA (right panel) shows the dissection flap involving the non-coronary sinus of Valsalva (arrowheads). Ascending thoracic aorta is dilated. Solid and empty arrows indicate right coronary artery and left main coronary artery, respectively. Another coronal oblique MPR image (left panel), obtained posteriorly to the right panel, demonstrates localized aortic dissection at the sinus of Valsalva (arrowheads).
E. Axial oblique MPR image in the follow up CCTA, which is reconstructed to correspond with the intra-operative picture (F), presents a dissection flap (arrowheads) that starts from the N and extends to the L.
F. Intra-operative picture shows the extensive dissection flap (arrowheads), extending from the N to the L..
AA = ascending thoracic aorta, CCTA = coronary computed tomography angiography, L = left coronary sinus, LCX = left circumflex coronary artery, MPR = multiplanar reformatted, N = non-coronary sinus, R = right coronary sinus
References
1. Batra P, Bigoni B, Manning J, Aberle DR, Brown K, Hart E, et al. Pitfalls in the diagnosis of thoracic aortic dissection at CT angiography. Radiographics. 2000; 20:309–320.
2. Roos JE, Willmann JK, Weishaupt D, Lachat M, Marincek B, Hilfiker PR. Thoracic aorta: motion artifact reduction with retrospective and prospective electrocardiography-assisted multi-detector row CT. Radiology. 2002; 222:271–277.
3. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000; 283:897–903.
4. Sommer T, Fehske W, Holzknecht N, Smekal AV, Keller E, Lutterbey G, et al. Aortic dissection: a comparative study of diagnosis with spiral CT, multiplanar transesophageal echocardiography, and MR imaging. Radiology. 1996; 199:347–352.
5. Zhao H, He B, Shen X, Qiao Z, Xu T, Lian F, et al. Aortic root dissection with left valsalva sinus perforation detected by transesophageal 3D echocardiography in a patient with Behcet's disease. J Clin Ultrasound. 2014; 42:59–62.
6. Manghat NE, Morgan-Hughes GJ, Roobottom CA. Multidetector row computed tomography: imaging in acute aortic syndrome. Clin Radiol. 2005; 60:1256–1267.
7. Bae JM, Choe YH, Hwang HW, Kim JS, Kim WS, Peck KR, et al. Tumor-mimicking large vegetation attached to the tricuspid valve without predisposing factors: a case report on CT and echocardiographic findings. J Korean Soc Radiol. 2015; 73:269–273.
8. Cigarroa JE, Isselbacher EM, DeSanctis RW, Eagle KA. Diagnostic imaging in the evaluation of suspected aortic dissection. Old standards and new directions. N Engl J Med. 1993; 328:35–43.
9. Zeman RK, Berman PM, Silverman PM, Davros WJ, Cooper C, Kladakis AO, et al. Diagnosis of aortic dissection: value of helical CT with multiplanar reformation and three-dimensional rendering. AJR Am J Roentgenol. 1995; 164:1375–1380.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download