Abstract
The most common site of metastasis in gestational trophoblastic disease (GTD) is the lung. To the best of our knowledge, arteriovenous malformations (AVMs) associated with pulmonary metastatic lesions are extremely rare in patients with GTD. Here, we report a case of a nineteen-year-old woman who presented with pulmonary AVMs which developed in the areas of pulmonary metastases, complicated by recent hemorrhage.
Gestational trophoblastic disease (GTD) comprises a broad spectrum of disorders that arise from uncontrolled growth of placental trophoblastic tissue, and hydatidiform mole is a pre-malignant lesion of GTD originating from aberrant fertilization (1). GTD refers to a type of tumor which is, characteristically, highly vascularized and can cause heavy or life-threatening bleeding (23). The development of uterine arteriovenous malformations (AVMs) can be found in patients with GTD because of abnormal trophoblastic proliferation and increased angiogenesis caused by high production of human chorionic gonadotropin (hCG) (4).
The primary site of metastasis in GTD is the lung, with an incidence of 76–87% (1). However, the formation of AVMs within pulmonary metastatic lesions is rare in patients with GTD. We are reporting on a case of AVMs which developed in the areas of pulmonary metastases before treatment in a patient with hydatidiform mole. This report was approved by the Institutional Review Board of our institution.
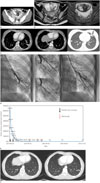
A nineteen-year-old woman visited our emergency room due to a febrile sensation and lower abdominal pain in June of 2014. She reported a four month history of vaginal bleeding. She was suspected of hydatidiform mole on pelvic sonography. On admission, the serum hCG level was 62200.02 mIU/mL. The findings on abdominal computed tomography (CT) and pelvic magnetic resonance imaging (MRI) revealed an enlarged uterus with abnormal heterogeneous enhancement, typical of molar pregnancy (Fig. 1A, B). At that time, chest radiography and contrast-enhanced chest CT scanning revealed multiple metastatic nodules in both lungs, and there were curvi-linear structures that were connected to some metastatic nodules in both lower lobes and the inferior lingular segment of the left upper lobe. Around the metastatic nodule, there were patchy ground-glass opacity lesions in the inferior lingular segment of the left upper lobe, consistent with pulmonary hemorrhage. The findings on chest CT consisted of multiple pulmonary metastases with AVMs, complicated by recent hemorrhage (Fig. 1C). No other metastasis was found in the brain or abdomen on abdominal CT or brain MRI. During admission, she was in septic shock (body temperature; 38.5℃, blood pressure; 80/60 mm Hg, heart rate; 120 beats per minute). After uterine embolization, dilatation and curettage was performed twice. Pulmonary arteriography confirmed the presence of two left AVMs and pulmonary AVMs were successfully embolized with a 2 × 5 mm coil one week later, as shown in Fig. 1D. Then, she was given three cycles of Methotrexate weekly and she attained a normal hCG level (Fig. 1E). She was regularly followed up and the findings on follow-up chest CT showed that multiple metastatic nodules with residual AVMs in both lungs had markedly decreased in size in January 2015 (Fig. 1F).
GTD is a rare entity, and it manifests as an hCG-producing hypervascular tumor (2). The key regulatory role of hCG in angiogenesis and vascular function is well known (4). AVMs in the uterus are a relatively frequent event, and the relationship between GTD and uterine AVMs is well-documented by abnormal trophoblastic proliferation and angiogenesis induced by high hCG production (245).
Pulmonary metastases of GTD are seen most often and are thought to be most common. Angiographic studies have shown that pulmonary metastases of GTD are hyper-vascular tumors, and are supplied by the pulmonary artery (6). Pulmonary AVMs are abnormally dilated vessels which provide an anatomic right-to-left shunt between the pulmonary artery and the pulmonary vein without an intervening pulmonary capillary system (7). Most pulmonary AVMs are congenital in etiology, and are associated with hereditary hemorrhagic telangiectasia. Acquired pulmonary AVMs can occur in the context of a variety of physical injuries or disease processes such as liver cirrhosis, chest trauma, infection, metastatic carcinoma, mitral stenosis or systemic amyloidosis (78). Pulmonary metastases of GTD have the potential to form pulmonary AVMs, which can cause life-threatening hemorrhage or hemoptysis (2). A few studies have shown that pulmonary AVMs were secondary to GTD at the metastatic site during or after chemotherapy (26910). Our patient showed multiple pulmonary metastases and AVMs within these lesions accompanied by pulmonary hemorrhage even before the onset of chemotherapy.
Uterine AVMs in patients with GTD have the potential risk of heavy vaginal bleeding or intraperitoneal hemorrhage resulting from disorganized vascular structures and high blood flow (5). Uterine or pulmonary AVMs may be spontaneously resolved with successful chemotherapy, and these patients were treated for persistent or life-threatening bleeding with embolization (35). However, AVMs might persist after successful completion of chemotherapy (2910). In our case, embolization was performed to reduce the bleeding risk, because our patient had pulmonary hemorrhage associated with pulmonary AVMs. In addition to metastatic nodules, residual pulmonary AVMs had disappeared or were markedly decreased with successful chemotherapy.
In conclusion, AVMs associated with pulmonary metastatic lesions are extremely rare. When the early diagnosis of pulmonary AVM using CT scanning is established, appropriate embolization and chemotherapy could be considered in an effort to reduce the risk of bleeding and to improve the outcome.
Figures and Tables
Fig. 1
A 19-year-old woman with hydatidiform mole, metastasizing to the lung accompanying with the pulmonary; arteriovenous malformation.
A. Contrast-enhanced abdomen CT shows an enlarged uterus with abnormal heterogeneous enhancement of the endometrium (white thick arrows). Multiple air bubbles are found in the uterine cavity because dilatation and curettage is performed on the same day.
B. Axial T2-weighted (left) and T1-weighted gadolinium-enhanced (right) pelvic MR images demonstrate strong enhancement (white thin arrows) of the endometrium with multiple T2-hyperintense cystic foci (a black thick arrow).
C. Axial contrast-enhanced chest CT shows multiple nodules consistent with pulmonary metastases. Some nodules communicate with a curvilinear structure (white thick arrows), indicating arteriovenous malformations. Ground-glass halo around a metastatic nodule in the inferior lingular segment of the left upper lobe represents hemorrhage (a black arrow).
D. Pulmonary angiography and coil embolization. Selective left pulmonary arteriogram shows pulmonary AVM (white thick arrows). Embolization coil (a black arrow) is deployed and postembolization pulmonary arteriogram shows complete occlusion of AVM.
AVM = arteriovenous malformation, CT = computed tomography
E. hCG levels during the course of treatment. At the time of diagnosis, the serum hCG level was 62,200.02 mIU/mL. The serum hCG level decreased to 3897.82 mIU/mL after two dilatation and curettage on June 26 and July 2, 2014. From July 28 to August 11, the patient was given three cycles of Methotrexate weekly. The serum hCG level decreased to 9.85 mIU/mL on first follow-up examination after Methotrexate administration. Thereafter, the serum hCG level had decreased continuously, and it was less than 0.5 mIU/mL in the last examination performed on July 6, 2016.
F. Follow-up contrast-enhanced chest computed tomography obtained after embolization and three courses of methotrexate shows that multiple metastatic nodules with residual arteriovenous malformations (white thick arrows) in both lungs have markedly decreased in size or disappeared (arrows).
hCG = human chorionic gonadotropin
Acknowledgments
The authors would like to thank the CT, MR, intervention technologists at the Department of Radiology, Kangwon National University Hospital.
References
1. Shanbhogue AK, Lalwani N, Menias CO. Gestational trophoblastic disease. Radiol Clin North Am. 2013; 51:1023–1034.
2. McDonald-Burrows Z, Davies R, Goode E, Clarke C, Jackson J, Seckl M, et al. Haemoptysis from a pulmonary arteriovenous malformation in a post molar pregnancy gestational trophoblast tumour patient managed by radiological embolisation: a case report. J Med Case Rep. 2014; 8:117.
3. McGrath S, Harding V, Lim AK, Burfitt N, Seckl MJ, Savage P. Embolization of uterine arteriovenous malformations in patients with gestational trophoblastic tumors: a review of patients at Charing Cross Hospital, 2000-2009. J Reprod Med. 2012; 57:319–324.
4. Zygmunt M, Herr F, Münstedt K, Lang U, Liang OD. Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2003; 110:Suppl 1. S10–S18.
5. Touhami O, Gregoire J, Noel P, Trinh XB, Plante M. Uterine arteriovenous malformations following gestational trophoblastic neoplasia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2014; 181:54–59.
6. Green JD, Carden TS Jr, Hammond CB, Johnsrude IS. Angiographic demonstration of arteriovenous shunts in pulmonary metastatic choriocarcinoma. Radiology. 1973; 108:67–70.
7. Shovlin CL. Pulmonary arteriovenous malformations. Am J Respir Crit Care Med. 2014; 190:1217–1228.
8. Pick A, Deschamps C, Stanson AW. Pulmonary arteriovenous fistula: presentation, diagnosis, and treatment. World J Surg. 1999; 23:1118–1122.
9. Casson AG, McCormack D, Craig I, Inculet R, Levin L. A persistent pulmonary lesion following chemotherapy for metastatic choriocarcinoma. Chest. 1993; 103:269–270.
10. Choi SH, Goo JM, Kim HC, Im JG. Pulmonary arteriovenous fistulas developed after chemotherapy of metastatic choriocarcinoma. AJR Am J Roentgenol. 2003; 181:1544–1546.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download