Abstract
Purpose
The aim of this study was to determine the radiologic risk factors of colorectal cancer (CRC) with synchronous liver metastases.
Materials and Methods
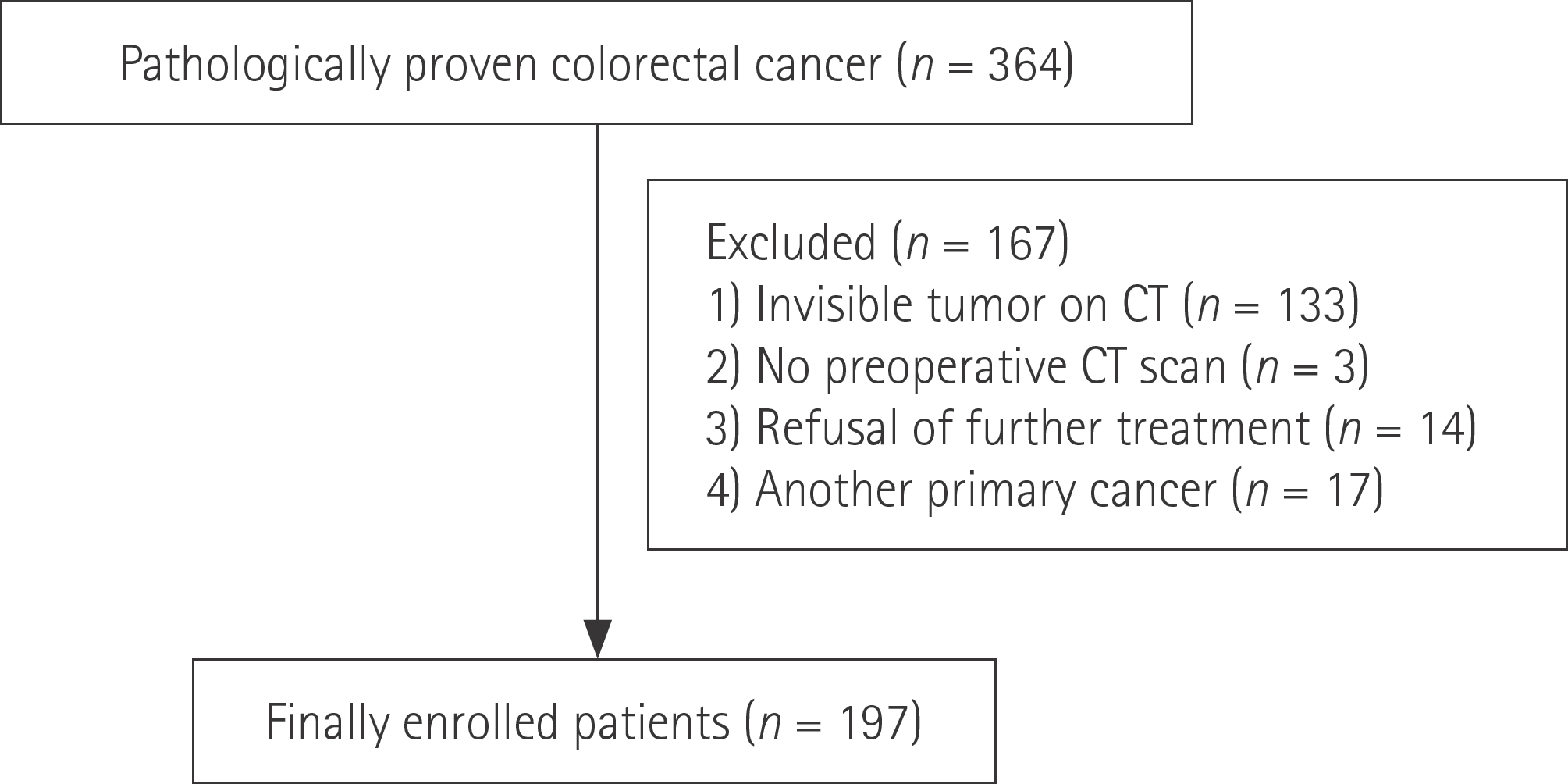
A total of 197 patients with CRC who had a visible tumor on contrast-enhanced abdominopelvic computed tomography and were treated be-tween January 2012 and December 2012 were included. Longitudinal diameter, mu-ral thickness, primary tumor attenuation, and other metastases were evaluated in-dependently. Univariate analysis and multivariate logistic regression analysis were used to identify risk factors associated with the presence of liver metastases.
Results
Cases were divided into two groups based on the presence or absence of liver metastases (n = 56 and 141, respectively). Primary tumors with enhancement of ≥ 90 Hounsfield units (HU) were found to have a higher risk of liver metastases than those with enhancement of < 90 HU [odds ratio (OR): 2.619, p = 0.034]. The presence of pulmonary metastases was associated with a higher risk of liver metas-tases (OR: 14.218, p = 0.025). The presence of lymph node metastases (N2 vs. N0) and carcinoembryonic antigen (CEA) level independently predicted the presence of liver metastases (OR: 8.766, p < 0.001; OR: 1.012, p = 0.048).
Go to : 
REFERENCES
1.Jemal A., Bray F., Center MM., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J Clin. 2011. 61:69–90.

2.Ferlay J., Shin HR., Bray F., Forman D., Mathers C., Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBO-CAN 2008. Int J Cancer. 2010. 127:2893–2917.

3.Mella J., Biffin A., Radcliffe AG., Stamatakis JD., Steele RJ. Pop-ulation-based audit of colorectal cancer management in two UK health regions. Colorectal Cancer Working Group, Royal College of Surgeons of England Clinical Epidemiology and Audit Unit. Br J Surg. 1997. 84:1731–1736.
4.Paschos KA., Bird N. Current diagnostic and therapeutic ap-proaches for colorectal cancer liver metastasis. Hippokratia. 2008. 12:132–138.
6.Welch JP., Donaldson GA. The clinical correlation of an au-topsy study of recurrent colorectal cancer. Ann Surg. 1979. 189:496–502.
7.Schmiegel W., Pox C., Reinacher-Schick A., Adler G., Arnold D., Fleig W, et al. S3 guidelines for colorectal carcinoma: re-sults of an evidence-based consensus conference on Febru-ary 6/7, 2004 and June 8/9, 2007 (for the topics IV, VI and VII). Z Gastroenterol. 2010. 48:65–136.
8.Kopetz S., Chang GJ., Overman MJ., Eng C., Sargent DJ., Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and im-proved chemotherapy. J Clin Oncol. 2009. 27:3677–3683.

9.Minami Y., Kudo M. Radiofrequency ablation of liver metas-tases from colorectal cancer: a literature review. Gut Liver. 2013. 7:1–6.

10.Tomlinson JS., Jarnagin WR., DeMatteo RP., Fong Y., Kornprat P., Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007. 25:4575–4580.

11.Abdalla EK., Adam R., Bilchik AJ., Jaeck D., Vauthey JN., Mahvi D. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006. 13:1271–1280.

12.Rees M., Tekkis PP., Welsh FK., O'Rourke T., John TG. Evaluation of long-term survival after hepatic resection for met-astatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008. 247:125–135.
13.Ye LC., Liu TS., Ren L., Wei Y., Zhu DX., Zai SY, et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol. 2013. 31:1931–1938.

14.Cremolini C., Loupakis F., Antoniotti C., Lupi C., Sensi E., Lonar-di S, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with meta-static colorectal cancer: updated overall survival and molec-ular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015. 16:1306–1315.

15.Hunter CJ., Garant A., Vuong T., Artho G., Lisbona R., Tekkis P, et al. Adverse features on rectal MRI identify a high-risk group that may benefit from more intensive preoperative staging and treatment. Ann Surg Oncol. 2012. 19:1199–1205.

16.Kim YC., Kim JK., Kim MJ., Lee JH., Kim YB., Shin SJ. Feasibility of mesorectal vascular invasion in predicting early distant metastasis in patients with stage T3 rectal cancer based on rectal MRI. Eur Radiol. 2016. 26:297–305.

17.Screening for synchronous metastases in colorectal cancer with DW-MRI (SERENADE). Available at:. https://clinicaltri-als.gov/ct2/show/NCT02246634. Published Sep 5, 2014. Ac-cessed Apr 12,. 2017.
18.Kim JW., Jeong YY., Chang NK., Heo SH., Shin SS., Lee JH, et al. Perfusion CT in colorectal cancer: comparison of perfusion parameters with tumor grade and microvessel density. Ko-rean J Radiol. 2012. 13(Suppl 1):S89–S97.

19.Russell AH., Harris J., Rosenberg PJ., Sause WT., Fisher BJ., Hoff-man JP, et al. Anal sphincter conservation for patients with adenocarcinoma of the distal rectum: long-term results of radiation therapy oncology group protocol 89-02. Int J Ra-diat Oncol Biol Phys. 2000. 46:313–322.

20.Mohiuddin M., Marks G., Bannon J. High-dose preoperative radiation and full thickness local excision: a new option for selected T3 distal rectal cancers. Int J Radiat Oncol Biol Phys. 1994. 30:845–849.

21.Gertler R., Rosenberg R., Schuster T., Friess H. Defining a high-risk subgroup with colon cancer stages I and II for possible adjuvant therapy. Eur J Cancer. 2009. 45:2992–2999.

22.Horton KM., Abrams RA., Fishman EK. Spiral CT of colon can-cer: imaging features and role in management. Radiograph-ics. 2000. 20:419–430.

23.Kim JE., Lee JM., Baek JH., Moon SK., Kim SH., Han JK, et al. Dif-ferentiation of poorly differentiated colorectal adenocarci-nomas from well- or moderately differentiated colorectal adenocarcinomas at contrast-enhanced multidetector CT. Abdom Imaging. 2015. 40:1–10.

24.Filippone A., Ambrosini R., Fuschi M., Marinelli T., Genovesi D., Bonomo L. Preoperative T and N staging of colorectal can-cer: accuracy of contrast-enhanced multi-detector row CT colonography--initial experience. Radiology. 2004. 231:83–90.

25.Sica GT., Ji H., Ros PR. CT and MR imaging of hepatic metas-tases. AJR Am J Roentgenol. 2000. 174:691–698.

26.Chung WS., Kim MJ., Chung YE., Kim YE., Park MS., Choi JY, et al. Comparison of gadoxetic acid-enhanced dynamic imaging and diffusion-weighted imaging for the preoperative evaluation of colorectal liver metastases. J Magn Reson Im-aging. 2011. 34:345–353.

27.Seo HJ., Kim MJ., Lee JD., Chung WS., Kim YE. Gadoxetate di-sodium-enhanced magnetic resonance imaging versus contrast-enhanced 18F-fluorodeoxyglucose positron emission tomography/computed tomography for the detection of colorectal liver metastases. Invest Radiol. 2011. 46:548–555.

28.Adam R., de Gramont A., Figueras J., Kokudo N., Kunstlinger F., Loyer E, et al. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consen-sus. Cancer Treat Rev. 2015. 41:729–741.

29.Goh V., Glynne-Jones R. Perfusion CT imaging of colorectal cancer. Br J Radiol. 2014. 87:20130811.

30.Weiss L., Grundmann E., Torhorst J., Hartveit F., Moberg I., Eder M, et al. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986. 150:195–203.
31.Viadana E., Bross ID., Pickren JW. The metastatic spread of cancers of the digestive system in man. Oncology. 1978. 35:114–126.

32.Weiss L., Voit A., Lane WW. Metastatic patterns in patients with carcinomas of the lower esophagus and upper rectum. Invasion Metastasis. 1984. 4:47–60.
33.Mantke R., Schmidt U., Wolff S., Kube R., Lippert H. Incidence of synchronous liver metastases in patients with colorectal cancer in relationship to clinico-pathologic characteristics. Results of a German prospective multicentre observational study. Eur J Surg Oncol. 2012. 38:259–265.

34.Kang KA., Jang KM., Kim SH., Kang TW., Cha DI. Risk factor as-sessment to predict the likelihood of a diagnosis of metas-tasis for indeterminate hepatic lesions found at computed tomography in patients with rectal cancer. Clin Radiol. 2017. 72:473–481.

35.Jones EC., Chezmar JL., Nelson RC., Bernardino ME. The fre-quency and significance of small (less than or equal to 15 mm) hepatic lesions detected by CT. AJR Am J Roentgenol. 1992. 158:535–539.

36.Krakora GA., Coakley FV., Williams G., Yeh BM., Breiman RS., Qayyum A. Small hypoattenuating hepatic lesions at contrast-enhanced CT: prognostic importance in patients with breast cancer. Radiology. 2004. 233:667–673.

37.Goshima S., Kanematsu M., Watanabe H., Kondo H., Shiratori Y., Onozuka M, et al. Hepatic hemangioma and metastasis: differentiation with gadoxetate disodium-enhanced 3-T MRI. AJR Am J Roentgenol. 2010. 195:941–946.

Go to : 
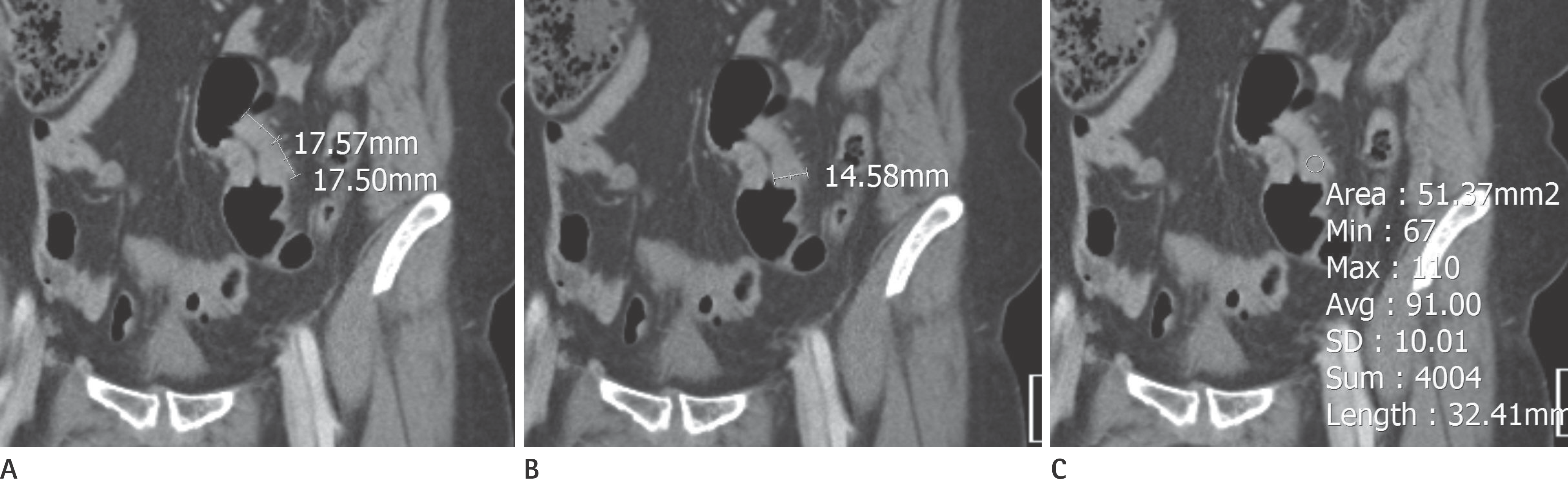 | Fig. 2.Proximal sigmoid colon cancer in a 58-year-old woman. (A) Longitudinal tumor diameter, (B) tumor mural thickness, and (C) tumor en-hancement (region of interest) of the primary cancer were measured. |
 | Fig. 3.Liver metastasis from recto-sigmoid colon cancer in a 67-year-old woman. (A) Transverse CT image shows a tiny indeterminate hypoat-tenuating lesion (arrow) in hepatic segment VI. (B) Transverse CT image shows a primary tumor (arrows). Tumor enhancement was 126 HU, sug-gestive of high risk. (C) The tiny lesion had increased in size as determined by follow-up CT 9 months later (arrow). (D) Gadoxetic acid-enhanced hepatobiliary phase image shows a hypointense lesion (arrow) in hepatic segment VI. (E) Diffusion weighted images (b = 1000 sec/mm2) and (F) T2-weighted images demonstrate a hyperintense lesion (arrows). This lesion was considered to be metastasis based on analyses of serial CT and magnetic resonance images. CT = computed tomography |
 | Fig. 4.Liver metastasis from recto-sigmoid colon cancer in an 80-year-old man. (A) Transverse CT did not depict a definite abnormal lesion in hepatic segment V. (B) Coronal reconstruction image shows a primary tumor. Its enhancement was 92 Hounsfield units, suggestive of high risk. Magnetic resonance images were obtained 3 days after initial CT. (C) Gadoxetic acid-enhanced hepatobiliary phase image shows a hypointense lesion (arrow) in hepatic segment V. (D) Diffusion weighted images (b = 800 sec/mm2) and (E) T2-weighted images demonstrate a hyperintense lesion (arrows). This lesion was confirmed to be metastasis by tumorectomy. CT = computed tomography |
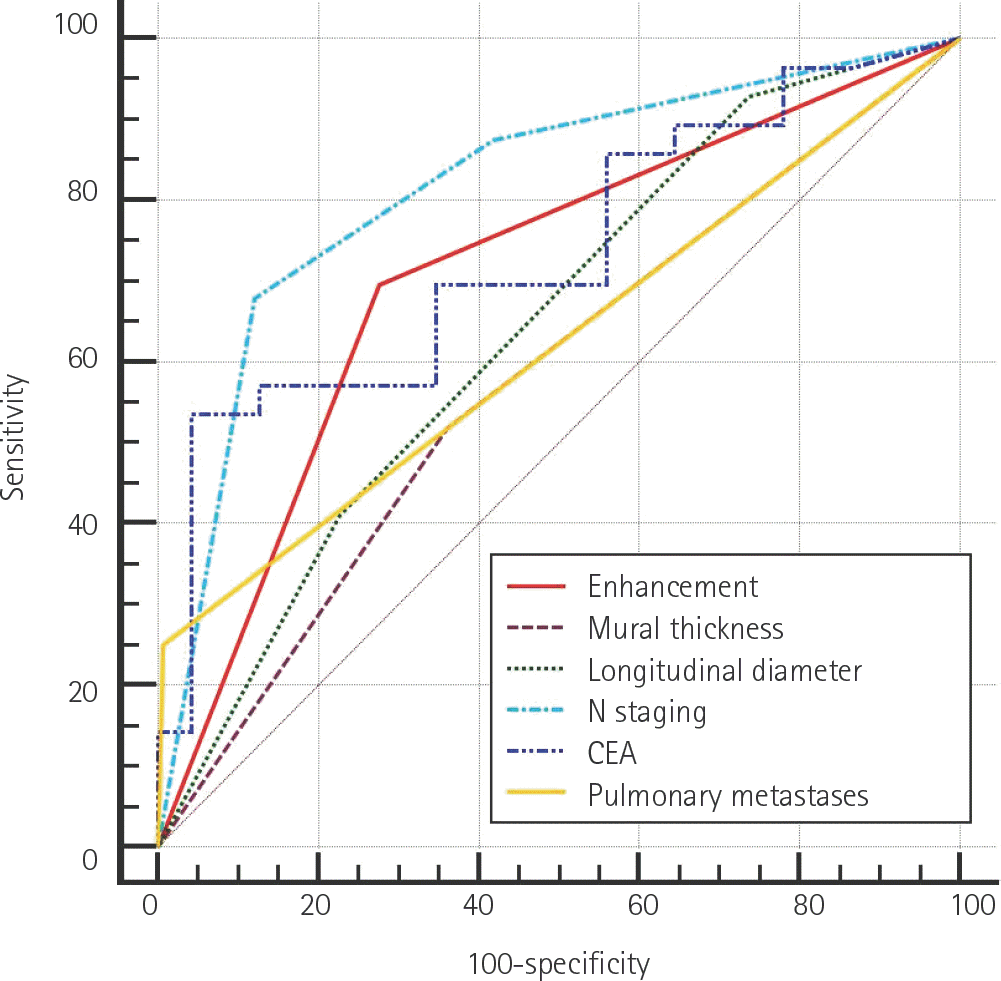 | Fig. 5.Receiver operating characteristic curve analysis results for fac-tors that were found to be positively associated with the presence of liver metastasis by logistic regression analysis. CEA = carcinoembryonic antigen |
Table 1.
Clinico-Pathological and Radiological Data of Colorectal Cancer Patients with or without Hepatic Metastases
| Variable | Patients with Liver Metastasis (n = 56) | Patients without Liver Metastasis (n = 141) | p-Value |
|---|---|---|---|
| Age | 66 ± 12.4 | 64 ± 12.8 | |
| Sex (M:F) | 33:23 | 89:52 | |
| Tumor location, n (%) | |||
| Right colon | 9 (16) | 32 (22.8) | |
| Left colon | 7 (12.5) | 24 (16.7) | |
| Rectosigmoid junction | 21 (37.5) | 44 (31.3) | |
| Rectal ampulla | 19 (34) | 39 (27.8) | |
| Synchronous | 0 | 2 (1.4) | |
| Cell type, n (%) | |||
| Adenocarcinoma | |||
| Well differentiated | 3 (5.4) | 12 (8.5) | |
| Moderate | 45 (80) | 115 (81.6) | |
| Poorly | 2 (3.6) | 3 (2.1) | |
| Mucinous adenocarcinoma | 5 (9.2) | 8 (5.7) | |
| Signet ring cell carcinoma | 1 (1.8) | 3 (2.1) | |
| pT staging | |||
| T1 | 0 | 6 (5) | |
| T2 | 0 | 24 (20) | |
| T3 | 24 (69) | 83 (69) | |
| T4 | 11 (31) | 7 (6) | |
| pN staging | |||
| N0 | 5 (14) | 81 (94.5) | |
| N1 | 11 (31) | 36 (3) | |
| N2 | 19 (55) | 3 (2.5) | |
| Unresectable condition, n (%) | 14 (25%, 14/56) | 8 (6%, 8/131) | |
| Neoadjuvant CRT, n (%) | 7 (13%, 7/56) | 13 (10%, 13/131) | |
| CEA (μg/L)* | 177.8 ± 593.3 | 10.4 ± 31.0 | 0.001 |
Table 2.
Characteristics of Liver Metastases
Table 3.
Radiological Finding of Primary Colorectal Cancer with or without Liver Metastases
| Variable | Patients with Liver Metastasis (n = 56) | Patients without Liver Metastasis (n = 141) | p-Value |
|---|---|---|---|
| Longitudinal diameter (mm)* | 54.9 ± 18.9 | 42.3 ± 19.5 | < 0.001 |
| Mural thickness (mm)* | 18.4 ± 8.8 | 14.4 ± 6.0 | 0.003 |
| Enhancement (ROI, HU)* | 91.4 ± 10.3 | 83.9 ± 11.1 | < 0.001 |
| Shape of tumor, n (%) | < 0.001 | ||
| Intraluminal polypoid | 0 (0) | 33 (23) | |
| Ulcerofungating or Ulceroinfiltrative pattern | 40 (71) | 102 (72) | |
| Bulky pattern | 16 (29) | 6 (5) | |
| Pericolic fat infiltration, n (%) | < 0.001 | ||
| None | 0 (0) | 33 (23) | |
| Hazy infiltration | 13 (23) | 36 (26) | |
| Linear infiltration | 29 (52) | 59 (42) | |
| Nodular infiltration | 14 (25) | 13 (9) | |
| Pulmonary metastases, n (%) | 14 (25) | 1 (0.7) | < 0.001 |
Table 4.
Risk Factors of Liver Metastases as Determined by Univari-ate Analysis
Table 5.
Risk Factors of Liver Metastases as Determined by Multi-variate Analysis
Table 6.
ROC Curve Analysis Results for Factors Identified by Logistic Regression Analysis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download