Abstract
Rhabdomyosarcoma mainly occurs in the pediatric age group, with the primary tumor originating from the trunk, neck, and extremities. Metastasis of rhabdomyosarcoma to the breast is very rare. Previous reports have suggested that the mammographic finding of breast rhabdomyosarcoma is an oval-shaped mass with irregular margins and the US finding is a solitary nodular lesion. We report a case of breast metastasis from pleural rhabdomyosarcoma in a 21-year-old woman, presenting as diffuse non-mass involvement and edematous change without a nodular mass.
Rhabdomyosarcoma (RMS) is a common malignancy in the pediatric age group, and its primary sites of origin are usually the trunk, neck, orbit, retroperitoneal space, and the extremities (1). Distant metastasis from RMS at diagnosis is common in lungs, bone marrow, bones, and distant lymph nodes (2). However, RMS is unusual in adults, and breast metastasis from RMS is very rare, accounting for less than 1% of all breast malignancies (1). There are several reports of imaging findings of breast metastasis from RMS. Here, we report a case of breast metastasis from pleural RMS with unusual imaging findings on ultrasonography (US).
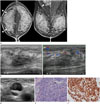
A 21-year-old woman presented to our breast unit with a palpable lump in the right breast. She had been diagnosed with alveolar RMS via pleural biopsy one week ago. On physical examination, there was a 60 mm-sized discrete palpable non-tender mass in the upper inner quadrant of the right breast. In the mammogram, global asymmetry with diffuse edematous change and enlarged ipsilateral axillary lymph nodes were observed (Fig. 1A). On US, there was a 56 mm-sized ill-defined hypoechoic lesion without discrete mass contour in the palpable site of the right breast (Fig. 1B). The lesion showed increased vascularity on Doppler US (Fig. 1C). A few enlarged lymph nodes with loss of fatty hilum were noted in levels I and II of the ipsilateral axilla (Fig. 1D). A 14 gauge US-guided core needle biopsy was performed for the palpable hypoechoic lesion in the right breast, and the lesion was confirmed as metastatic RMS. The tumor showed an infiltrative and solid growth pattern microscopically. Tumor cells had hyperchromatic nuclei and abundant cytoplasm (Fig. 1E). Immunohistochemical staining revealed diffuse positivity for desmin and negativity for anti-pan cytokeratin antibody (Fig. 1F).
The breast is an uncommon site for metastasis from extramammary malignancies. In a report by Mun, common radiologic features according to the hematogenous or lymphatic metastasis routes are well known. In case of hematogenous metastasis, it is located superficially due to the need for rich vascularity. Single or multiple hypoechoic masses without calcifications or secondary skin or nipple changes are the most well known US findings. Axillary lymph node metastasis is not a common finding. Lymphatic metastasis to the breast shows diffuse trabecular thickening and dense stroma without a primary mass (3).
RMS is a common primary malignancy with aggressiveness in the pediatric age group. Breast metastasis from RMS is uncommon with an incidence of 6% (4). Most of the cases occur in adolescent females, and the most common primary site is the extremity. Breast metastasis from pleural RMS is extremely rare (5). The longest survival is known to be 16 months from the first diagnosis due to a poor prognosis (6).
Because breast metastasis of RMS is commonly occurred in young women, US is more suitable than mammogram for the initial diagnostic imaging modality. A few previous reports suggested that metastatic RMS commonly show solitary nodular lesion, and less common in diffuse involvement or multiple lesion (7). In a report by Ahn et al. (8), heterogeneous echo pattern with poor vascularity on US was reported. Yang et al. (9) reported diffuse nodular infiltration without normal breast architecture. In our case, however, there was an ill-defined hypoechoic lesion at the palpable site and adjacent diffuse edema in the affected breast on US with no typical imaging feature of metastatic RMS as solitary nodule or discrete mass. In addition, a round and high density mass was reported as a typical mammographic features of metastatic RMS in the breast (10). According to another rarely known reports, circumscribed oval shaped masses with irregular margins are reported (4). It is not common to find microcalcifications, spiculation, architectural distortion or skin thickening (10). However, we report global asymmetry and edematous change along the breast with axillary lymphadenopathy on a mammogram.
In summary, we reported an extremely rare case of breast metastasis from pleural RMS. Various imaging findings of metastatic RMS have been reported (4). However, our case showed another new imaging feature of metastatic RMS in the breast including an ill-defined hypoechoic lesion with adjacent breast edema. Our case report suggests that it is important to be aware of the possibility of breast metastasis in patients with primary RMS although there is no typical discrete mass lesion on US. If there is an ill-defined hypoechoic lesion in the symptomatic area of the breast, pathologic confirmation should be performed to exclude the possibility of breast metastasis in a patient with primary RMS.
Figures and Tables
Fig. 1
Breast metastasis from rhabdomyosarcoma in a 21-year-old female.
A. Mammogram reveals global asymmetry (empty arrow) with diffuse skin thickening (arrowheads) in the right breast and several enlarged lymph nodes in the right axilla (white arrow).
B, C. US reveals an ill-defined hypoechoic lesion (white arrow) with overlying skin thickening (black arrow) in the palpable site of the right breast. Increased vascularity around the lesion is demonstrated.
D. There are a few enlarged lymph nodes with loss of fatty hilum in the right axilla.
E. Microscopic section of the right breast mass. It shows diffuse growth pattern with small round cell composition. Tumor cells have hyperchromatic nuclei and abundant cytoplasm (arrowhead) (hematoxylin-eosin stain, × 400).
F. Tumor cells immunostained for desmin shows a strong reaction (arrow).
References
1. Jung SP, Lee Y, Han KM, Lee SK, Kim S, Bae SY, et al. Breast metastasis from rhabdomyosarcoma of the anus in an adolescent female. J Breast Cancer. 2013; 16:345–348.
2. D'Angelo P, Carli M, Ferrari A, Manzitti C, Mura R, Miglionico L, et al. Breast metastases in children and adolescents with rhabdomyosarcoma: experience of the Italian Soft Tissue Sarcoma Committee. Pediatr Blood Cancer. 2010; 55:1306–1309.
3. Mun SH, Ko EY, Han BK, Shin JH, Kim SJ, Cho EY. Breast metastases from extramammary malignancies: typical and atypical ultrasound features. Korean J Radiol. 2014; 15:20–28.
4. Birjawi GA, Haddad MC, Tawil AN, Khoury NJ. Metastatic rhabdomyosarcoma to the breast. Eur Radiol. 2001; 11:555–558.
5. Perlet C, Sittek H, Forstpointner R, Kessler M, Reiser M. Metastases to the breast from rhabdomyosarcoma: appearances on MRI. Eur Radiol. 1999; 9:1113–1116.
6. Yang WT, Kwan WH, Chow LT, Metreweli C. Unusual sonographic appearance with color Doppler imaging of bilateral breast metastases in a patient with alveolar rhabdomyosarcoma of an extremity. J Ultrasound Med. 1996; 15:531–533.
7. Binokay F, Soyupak SK, Inal M, Celiktas M, Akgül E, Aksungur E. Primary and metastatic rhabdomyosarcoma in the breast: report of two pediatric cases. Eur J Radiol. 2003; 48:282–284.
8. Ahn SJ, Kim SK, Kim EK. Metastatic breast cancer from rhabdomyosarcoma mimicking normal breast parenchyma on sonography. J Ultrasound Med. 2010; 29:489–492.
9. Yang WT, Metreweli C. Sonography of nonmammary malignancies of the breast. AJR Am J Roentgenol. 1999; 172:343–348.
10. Sheen-Chen SM, Eng HL, Ko SF. Metastatic rhabdomyosarcoma to the breast. Anticancer Res. 2005; 25:527–529.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download