Abstract
Purpose
To determine prognostic factors for the development of liver abscess formation in patients with hepatocellular carcinoma (HCC) treated with radiofrequency ablation (RFA) focusing on the history of multiple prior sessions of transarterial chemoembolization (TACE).
Materials and Methods
Patients were included if RFA was performed from January 2005 to July 2016 for a single HCC nodule smaller than or equal to 5 cm or up to three nodules with each nodule smaller than or equal to 3 cm. Univariate and multivariate logistic regression analyses were conducted and propensity score matching was performed between those without multiple prior sessions of TACE before ablation (Group 1) and those with such a history (Group 2).
Results
Overall, 694 patients were included in this study. Liver abscesses were developed in four patients, all in Group 2. After performing 2:1 propensity score matching, 149 and 81 patients were selected for Group 1 and 2, respectively. Among these matched patients, rates of liver abscess development were 0% and 5.1% in Group 1 and 2, respectively. The difference in rate of liver abscess development between the two groups was statistically significant (p = 0.014).
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide. It commonly occurs in patients at high risk of this disease, especially those with cirrhosis (1). Radiofrequency ablation (RFA) is a widely practiced nonsurgical treatment modality for HCC patients (23456). Although RFA is less invasive than surgical therapies, major complications can occur, although infrequently. Liver abscess is one of the most common major complication (7). Previous studies have shown that pre-existing biliary abnormality is a potent predictor for the development of liver abscess (78910). However, factors other than biliary abnormalities have not been sufficiently elucidated yet to the best of our knowledge.
In a previous study, prior transarterial chemoembolization (TACE) with iodized oil uptake has been identified as an independently significant adverse prognostic factor for formation of liver abscess in patients after RFA (8). However, that study had a major weak point in that it did not include a control group. In contrast, other previous studies have reported that combined treatment of HCC with TACE and RFA does not increase the rate of major complications including liver abscess compared to RFA monotherapy (11). The objective of this study was to determine prognostic factors for the development of liver abscess after adjusting selection bias by propensity score matching focusing on the impact of multiple prior sessions of TACE.
Informed consent was obtained from all patients after the nature of procedures was fully explained. The Institutional Review Board in our hospital approved data collection and analysis for this study. Patients were included in this study if percutaneous RFA was performed in our center from January 2005 to July 2016. When patients had a single nodular HCC less than or equal to 5 cm or multiple nodules up to three in number with each nodule less than or equal to 3 cm and when surgical resection candidates showed clinically significant portal hypertension (i.e., the presence of varices or splenomegaly associated with thrombocytopenia), RFA was recommended as an alternative treatment modality instead of hepatic resection at our institute (12). In addition, RFA was performed based on the preference of patients against strong recommendation for surgery by clinicians. Patients who had been repeatedly treated with RFA at least 3 months from the previous ablation were regarded as new patients in this study. TACE was often performed prior to RFA when liver tumors were less conspicuous on ultrasonography or when the tumors were located in difficult locations for RFA (13).
The diagnosis of HCC was made using noninvasive criteria defined by the American Association for the Study of Liver Disease (AASLD) recommendations consisting of arterial hyper-enhancement with washout on portal- or delayed-phase images on gadoxetic acid-enhanced liver MRI and/or dynamic CT (1214). In patients who did not meet the non-invasive diagnostic criteria, HCC was diagnosed based on biopsy-proven pathological confirmation.
Percutaneous RFA was performed for inpatients under conscious sedation using a combination of intravenous fentanyl citrate (Fentanyl citrate®; Myungmoon Pharm. Co., Hwaseong, Korea) and midazolam (Midazolam; Bukwang Pharm. Co., Ansan, Korea) (15). Pre-ablation prophylactic antibiotics were not used. RFA device was selected and used in each procedure depending on the size and location of the tumor. Multitined expandable electrodes or internally cooled electrodes (single or multiple) were used as appropriate according to tumor size and location. All sonographic procedures were performed with a 3.5-MHz convex-array transducer using a free-hand technique. Percutaneous RFA was mostly performed with real-time sonographic guidance by two experienced radiologists (Y.K.C. and M.Y.K.) with eight years of experience in sonography guided ablation procedures at the start of this study. When sonographic guidance was not technically feasible, CT guidance was used. The ablation procedure was terminated when the size of ablation zone on US monitoring was large enough to achieve at least 5 mm of safety margin (2). Vital signs were monitored during the entire procedure.
TACE procedure was performed by two interventional radiologists (Y.K.C. and Y.S.A. with experience of over seven years and four years at the start of this study, respectively). Hepatic angiography was performed using 5 Fr angiographic catheters followed by superselection of tumor feeders as distal as possible using a microcatheter. An iodized oil-doxorubicin hydrochloride (Adriamycin; Dong-A Pharm., Seoul, Korea) emulsion was then administered into the feeders. The volume of iodized oil ranged from 3 to 10 mL. Once the flow became sluggish, gelatin sponge particles (Cutanplast; Mascia Brunelli, Italy) mixed with contrast material were administered into feeders until blood flow was diminished to minimum level.
CT examinations were performed with 16-, 64-, or 256-slice multidetector CT scanners. MR images were obtained from either a 1.5T or a 3.0T superconducting system using an 8-channel or a 32-channel phased-array coil, respectively. The number of tumors was determined on pretreatment CT or MR imaging. Tumor size was determined as the maximal diameter of the tumor measured on pre-ablation CT or MRI images taken within one month from the ablation procedure.
Major complications were defined as those that might threaten the patient's life, lead to substantial morbidity and disability, or result in lengthened hospital stay. All other complications were considered minor (16). Treatment mortality was defined as any death within 30 days after RFA. Information was extracted from a prospective database for RFA by a radiologist with 15 years of experience in liver imaging. Another senior radiologist with 20 years of experience in liver imaging interpreted CT or MR images with positive or equivocal findings of liver abscess. Final decision was made by consensus.
To determine prognostic factors of liver abscess formation, univariate and multivariate analyses were performed. Parameters that proved to be significant or marginally significant (p-value < 0.1) in univariate analysis were subsequently tested in the multivariate logistic regression model. To adjust for potential bias, propensity score matching was carried out between those treated without a history of multiple prior sessions of TACE in the same segment before RFA (Group 1) and those with such a history (Group 2) using chi-squared test. Propensity score matching was performed for 21 potential factors, including age greater than or equal to 65 years, sex, multinodularity, largest tumor greater than or equal to 3 cm, presence of a centrally located tumor, a dome nodule, a subcapsular tumor, adjacent gastrointestinal tract or adjacent large intrahepatic vessel greater than or equal to 3 mm, use of internally cooled single electrode, use of multiple expandable electrodes, CT guidance, Child-Pugh class, hepatitis C viral infection, alcoholics, coexistence of other malignancy, diabetes mellitus, and hypertension. A caliper of 0.03 was applied for propensity score matching. For matched patients, differences in incidence of liver abscess formation and major complications were compared between the two groups using chi-squared test. Statistically significance was considered at p < 0.05.
From January 2005 to July 2016, 694 patients met our inclusion criteria, including 603 patients in Group 1 and 91 patients in Group 2. Baseline demographic and tumor characteristics are summarized in Table 1. The two groups showed significant differnces in five potential prognostic factors, including multinodularity (p = 0.020), liver dome nodule (p = 0.036), diabetes mellitus (p = 0.000), CT-guided ablation (p = 0.000), and repeated sessions of ablation (p = 0.011). Among those 91 patients in Group 2, 44, 18, and 29 patients had two, three, and four or more prior TACE sessions, respectively. Liver abscesses were developed in four (0.58%) patients, all of which were in Group 2 (Fig. 1). The rate of liver abscess formation was 0% in Group 1 and 4.4% (p < 0.001) in Group 2. All four liver abscesses were developed after ultrasonography-guided RFA. They were conservatively managed with the administration of intravenous antibiotics and percutaneous drainage catheter insertion. Liver abscess developed at 5, 6, 24, or 75 days after RFA in these four patients. Three of the four liver abscesses were developed in patients who received repeated sessions of ablation for local control of index tumors.
Major complications occurred in 18 (2.6%) patients, including 13 patients in Group 1 and 5 patients in Group 2. Major complications other than liver abscess included hepatic or its parasitic arterial bleeding (3 patients), gastrointestinal tract injury (2 patients), major bile duct injury (2 patients), hepatic failure (3 patients), right pleural effusion (1 patient), hemothorax (1 patient), localized peritonitis (1 patient), and finally pneumoperitoneum (1 patient). The rate of major complications was 2.2% in Group 1 and 5.5% in Group 2. The difference between the two groups was not statistically significant (p = 0.175).
Univariate analysis of prognostic factors showed that only repeated sessions of ablation were statistically significant for liver abscess formation (p = 0.018) (Table 2). Hazard ratios of several potential prognostic factors such as post-endoscopic retrograde cholangiopancreaticography (ERCP) status, adjacent large intrahepatic vessel, adjacent gastrointestinal tract, CT-guided ablation, or history of previous TACE could not be properly investigated using logistic regression analysis because all liver abscesses were developed only in one arm related to each factor. Fisher's exact test revealed that prior session of TACE before ablation was the only statistically significant (p < 0.001) predictor among these factors for liver abscess formation.
After performing 2:1 propensity score matching, 149 patients in Group 1 and 79 patients in Group 2 were selected. The two groups were comparable with each other for all potential prognostic factors (Table 3). Among these matched patients, liver abscess occurred in four patients, all of them were in Group 2. The rate of liver abscess development was 0% in Group 1 and 5.1% in Group 2. Their difference was statistically significant (p = 0.014). Major complications other than liver abscess included acute arterial bleeding (2 patients), acute kidney injury (1 patient), and hepatorenal syndrome (1 patient) in Group 1 and hepatic failure (1 patient) in Group 2. The overall major complication rate in Group 1 was not statistically different from that in Group 2 (2.7% vs. 6.3%; p = 0.281).
It is well known that liver abscess formation is the most common major complication in HCC patients following RFA, with rates ranging from 0.67% to 2.0% (781718). The rate of liver abscess formation in this study was 0.58%, similar to results of previous studies. It has been reported that biliary abnormalities caused by biliary surgery or ERCP are potent predictors for liver abscess formation (81920). However, no such association was found in this study. Such difference might be due to the fact that there was no patient with a prior history of biliary surgery in this study. In addition, liver abscess did not develop among the six patients who had prior history of ERCP. The effect of ERCP on the occurrence of liver abscess needs to be evaluated in a future large-scale study.
After adjusting selection bias, the rate of liver abscess development after RFA for HCC was higher in patients with a history of repeated TACE in the same segment prior to RFA compared to that in patients without such a history. The rate of 5.5% in Group 2 exceeded the upper limit of liver abscess formation rate reported in the literature. In contrast, previous studies have reported that combined treatment of HCC with TACE and RFA does not increase the rate of major complications including liver abscess compared to RFA monotherapy (11). The frequency of liver abscess formation after TACE monotherapy is also shown to be very rare (21). Such contradictory results might be due to differences in the number of TACE sessions in the same segment prior to RFA. In this study, all four patients with liver abscess after RFA had prior history of multiple sessions of TACE.
In a previous study, prior TACE with iodized oil uptake, but not TACE itself, has been found to be a significant adverse risk factor for the development of liver abscess after RFA (8). The exact pathophysiology of TACE in the development of liver abscess remains unclear. However, it has been found that TACE can cause bile duct necrosis in 12.5% of patients (2223). The biliary tree is supplied primarily by peribiliary capillary plexus, a vascular plexus of hepatic arterial branches surrounding bile ducts. Biliary duct injuries caused by repeated TACE may provide a plausible explanation for the increased risk of hepatic abscess formation after RFA. Biliary injury following repeated TACE and subsequent thermal injury caused by RFA might have predisposed patients to the development of liver abscess (8). The fact that three of four liver abscesses were developed in patients who received repeated sessions of ablation for local control of index tumors suggests that repeated ablation might increase the rate of liver abscess formation. Nevertheless, after propensity score matching, repeated session of ablation lost its statistical significance as a prognostic factor for liver abscess formation.
In contrast to results of a previous study (8), the use of internally cooled single electrodes was not found to be a predisposing factor for the development of liver abscess in this study. The exact mechanism of this difference is not definite. It might be due to the fact that the ablation power was steadily increased from 60 W up to 150 W in our center, while a constant power of 200 W was applied in the previous study (8). Such modified ablation protocol might have caused less destructive thermal injury to peritumoral tissue. Old age has been found to be a significant prognostic factor for liver abscess formation in a prvious study (24). However, this was not observed in this study. Currently there is no reasonable explanation for old age to be a risk factor for for liver abscess formation.
This study has several limitations. First, there were only four patients with liver abscess in this study. Therefore, the strength of the study might be limited. A future multicenter study is needed to enhance the strength of evidence. Second, selection bias between groups might have been substantial because of its retrospective nature. However, it was adjusted by performing propensity score matching. Third, the frequency of overall major complication rates was not statistically significant despite difference in frequency of liver abscess formation.
In conclusion, history of multiple sessions of TACE in the same segment as index HCC nodule was found to be a potent predisposing factor for the development of liver abscess after performing RFA. Repeated ablation might also be a risk factor for liver abscess formation. However, further studies are required to confirm these findings.
Figures and Tables
Fig. 1
A 72-year-old male patient with hepatocellular carcinoma.
A. Pre-treatment post-contrast portal phase CT image showing a 2.5 cm sized hepatic nodule (arrow) diagnosed histopathologically as hepatocellular carcinoma.
B. Post-contrast CT scan performed one month after two repetitive transarterial chemoembolization showing a large liver abscess at the same site which was resolved with conservative management.
C. Post-contrast CT scan performed nine months after the last transarterial chemoembolization demonstrating a local tumor progression at the previous ablation site (arrow). Radiofrequency ablation was again performed to treat the local tumor progression.
D. Post-contrast CT scan performed one week after performing radiofrequency ablation showing a liver abscess (arrow) at the ablation site, extending into adjacent abdominal wall. The abscess was resolved with conservative management.
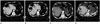
Table 1
The Baseline Characteristics of Patients

Patients were divided into those treated without multiple prior sessions of TACE before ablation (Group 1) and those with such a history (Group 2). There were 603 and 91 patients in Group 1 and 2, respectively.
*Statistically significant.
ERCP = endoscopic retrograde cholangiopancreaticography, HCC = hepatocellular carcinoma, RFA = radiofrequency ablation, TACE = transarterial chemoembolization
Table 2
Univariate Analysis of Prognostic Factors for the Formation of Liver Abscess after Radiofrequency Ablation of HCC

The other factors listed in Table 1 were not described in this table, because liver abscess developed in only one arm for the factors.
*Statistically significant.
HCC = hepatocellular carcinoma
Table 3
The Comparison of Baseline Characteristics of Matched Patients between Those Treated without Multiple Prior Sessions of TACE before Ablation and Those with Such a History

References
1. El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999; 340:745–750.
2. Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012; 107:569–577. quiz 578.
3. Peng ZW, Lin XJ, Zhang YJ, Liang HH, Guo RP, Shi M, et al. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology. 2012; 262:1022–1033.
4. Nakazawa T, Kokubu S, Shibuya A, Ono K, Watanabe M, Hidaka H, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol. 2007; 188:480–488.
5. Lee DH, Lee JM, Lee JY, Kim SH, Yoon JH, Kim YJ, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014; 270:900–909.
6. Kei SK, Rhim H, Choi D, Lee WJ, Lim HK, Kim YS. Local tumor progression after radiofrequency ablation of liver tumors: analysis of morphologic pattern and site of recurrence. AJR Am J Roentgenol. 2008; 190:1544–1551.
7. de Baère T, Risse O, Kuoch V, Dromain C, Sengel C, Smayra T, et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol. 2003; 181:695–700.
8. Choi D, Lim HK, Kim MJ, Kim SJ, Kim SH, Lee WJ, et al. Liver abscess after percutaneous radiofrequency ablation for hepatocellular carcinomas: frequency and risk factors. AJR Am J Roentgenol. 2005; 184:1860–1867.
9. Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002; 89:1206–1222.
10. Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003; 226:441–451.
11. Morimoto M, Numata K, Kondo M, Moriya S, Morita S, Maeda S, et al. Radiofrequency ablation combined with transarterial chemoembolization for subcapsular hepatocellular carcinoma: a prospective cohort study. Eur J Radiol. 2013; 82:497–503.
12. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012; 379:1245–1255.
13. Kim JH, Won HJ, Shin YM, Kim SH, Yoon HK, Sung KB, et al. Medium-sized (3.1-5.0 cm) hepatocellular carcinoma: transarterial chemoembolization plus radiofrequency ablation versus radiofrequency ablation alone. Ann Surg Oncol. 2011; 18:1624–1629.
14. Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005; 42:1208–1236.
15. Lang EV, Chen F, Fick LJ, Berbaum KS. Determinants of intravenous conscious sedation for arteriography. J Vasc Interv Radiol. 1998; 9:407–412.
16. Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. J Vasc Interv Radiol. 2014; 25:1691–1705.e4.
17. Mori K, Fukuda K, Asaoka H, Ueda T, Kunimatsu A, Okamoto Y, et al. Radiofrequency ablation of the liver: determination of ablative margin at MR imaging with impaired clearance of ferucarbotran--feasibility study. Radiology. 2009; 251:557–565.
18. Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003; 23:123–134. discussion 134-136.
19. Song SY, Chung JW, Han JK, Lim HG, Koh YH, Park JH, et al. Liver abscess after transcatheter oily chemoembolization for hepatic tumors: incidence, predisposing factors, and clinical outcome. J Vasc Interv Radiol. 2001; 12:313–320.
20. Kim W, Clark TW, Baum RA, Soulen MC. Risk factors for liver abscess formation after hepatic chemoembolization. J Vasc Interv Radiol. 2001; 12:965–968.
21. Kim MH, Choi MS, Choi YS, Kim DY, Lee JM, Paik SW, et al. Clinical features of liver abscess developed after radiofrequency ablation and transarterial chemoembolization for hepatocellular carcinoma. Korean J Hepatol. 2006; 12:55–64.
22. Kobayashi S, Nakanuma Y, Terada T, Matsui O. Postmortem survey of bile duct necrosis and biloma in hepatocellular carcinoma after transcatheter arterial chemoembolization therapy: relevance to microvascular damages of peribiliary capillary plexus. Am J Gastroenterol. 1993; 88:1410–1415.
23. de Baère T, Roche A, Amenabar JM, Lagrange C, Ducreux M, Rougier P, et al. Liver abscess formation after local treatment of liver tumors. Hepatology. 1996; 23:1436–1440.
24. Chen C, Chen PJ, Yang PM, Huang GT, Lai MY, Tsang YM, et al. Clinical and microbiological features of liver abscess after transarterial embolization for hepatocellular carcinoma. Am J Gastroenterol. 1997; 92:2257–2259.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download