Abstract
Primary presacral neuroendocrine tumor (NET) is extremely rare. Furthermore, its preoperative diagnosis is very difficult, and its imaging characteristics are not well described. We report the case of a 70-year-old female with presacral NET, and describe its imaging features on diffusion-weighted magnetic resonance imaging.
Neuroendocrine tumor (NET) is a rare neoplasm arising from enterochromaffin cells, and it is usually found in the gastrointestinal tract (approximately 65%), bronchopulmonary tract (approximately 25%), pancreas, or thyroid (12). However, presacral NET is extremely rare. A review of the literature revealed only 31 reported cases of primary presacral NET, and a few cases have described its imaging features. We present a case of presacral NET with a focus on magnetic resonance imaging (MRI) findings.
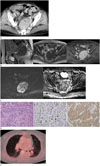
A 70-year-old woman was referred to our department of obstetrics and gynecology for an incidental presacral mass detected during a pelvic ultrasound. She had a surgical history of hysterectomy for uterine myoma. Laboratory examinations were within normal limits, and she had no symptoms, such as abdominal pain, vomiting, or a change in bowel habits. Contrast-enhanced computed tomography (CT) perfomed at the external institution revealed an 8 cm-sized presacral mass that abutted the sacrum. The mass was well-defined, oval, and solid with heterogeneous enhancement, and it contained an internal cystic portion and tiny calcifications (Fig. 1A).
Pelvic MRI was performed to evaluate the uncertain presacral mass. On MRI, the mass was located anterior to the sacrum, superior to the rectal shelf, and closely juxtaposed to the left piriformis muscle (Fig. 1B). The mass tightly abutted the sacrum causing resultant pressure erosion. However, fat planes between the mass and other neighborhood structures except for the sacrum were well preserved. The mass exhibited heterogeneous intermediate signal intensity on T2-weighted images, low signal intensity on T1-weighted images, and bright enhancement after intravenous contrast administration (Fig. 1B). Focal hyperintense foci within the mass were detected on T1- and T2-weighted images, indicating hemorrhage (Fig. 1B). Diffusion-weighted image (DWI) at a b value of 1000 s/mm2 revealed a hyperintense mass with a low apparent diffusion coefficient (ADC) value (mean, 0.632 × 10−3 mm2/sec), suggestive of a highly cellular tumor (Fig. 1C).
The patient underwent presacral mass resection with pelvic lymph node dissection. During surgery, the tumor was found to be hard and it was fixed to the sacrum, left pelvic side wall, and pelvic floor, and resultantly, it was incompletely removed. Grossly, several fragments of yellow tan colored soft tissues were identified. Microscopically, these tissues predominantly consisted of tumor cells with an infiltrative border in the background of fibrotic stroma. Analysis of tumor cells showed that it was a grade 2 NET, characterized by monomorphic, plasmacytoid neuroendocrine cells, with insular, solid and trabecular growth patterns (Fig. 1D). Immunohistochemical examination revealed positivity for the neuroendocrine marker (synaptophysin) and approximately 15% increase in the Ki-67 labeling index (Fig. 1D). Tumor tissues were negative for transcription factor 1, which differentiated metastatic tumor from pulmonary NET. The mass was finally diagnosed as a primary presacral well-differentiated grade 2 NET.
After presacral mass resection, positron emission tomography-CT was performed for staging of the NET. Multiple small pulmonary nodules with little 18F-fluorodeoxyglucose uptake were suspected to be metastatic lesions (Fig. 1E). There was no clinical progression during the 12-month observation period.
The presacral space is a clinically important space that contains multiple embryological remnants and lies at the intersection of the axial skeleton, neural axis, and pelvic soft tissues. Therefore, various benign or malignant conditions may occur in this space, including giant cell tumor, Ewing's sarcoma, osteosarcoma, chondrosarcoma, neurofibroma, paraganglioma, schwannoma, chordoma, hemangioma, myelolipoma, liposarcoma, germ cell tumor, congenital or developmental cysts, and metastasis (3). Involvement of the sacrum (either remodeling or destruction) and the presence or absence of a solid component may help narrow the differential diagnosis. Osteochondral and neurogenic tumors remodel or destroy the sacrum, whereas sacral involvement is less common in patients with mesenchymal tumors. Unilocular or multilocular purely cystic lesions without internal enhancement may suggest a congenital or developmental cyst.
Primary NET in the presacral space is extremely rare such that its preoperative diagnosis is very difficult. Presacral NET usually shows female predominance. Histopathologically, presacral NET shares features with NET of the rectum and other hindgut derivatives. Thus, its clinical features may be similar to those of rectal NET, which also tends to be silent (4). Presacral NET is usually asymptomatic and produces symptoms only related to its mass effect, such as pelvic pain, rectal fullness, and constipation. Presacral NET usually does not present symptoms of carcinoid syndrome, such as flushing, sweating, and hypertension, even in the presence of metastasis (4). Because presacral NET may be derived from the hindgut, it is associated with anomalies, such as tailgut cyst, presacral teratoma, imperforate anus, and Currarino syndrome (145).
Although imaging modalities have a limited role in the diagnosis of presacral NET, MRI may offer several advantages over CT. To date, MRI findings of presacral NET have been published previously in three case reports (467). MRI may help to characterize presacral NET by narrowing the differential diagnosis of presacral masses. In previous literatures, two cases presented as a predominantly solid mass and one case presented as a cystic mass (467). These solid masses appeared as relatively well-defined, lobulated, or infiltrative soft tissue masses with intermediate signal intensity on T2-weighted images, and they were well enhanced. Tiny calcifications and foci of hemorrhagic necrosis were observed in these masses (467). These imaging features were quite similar to those observed in our case. Moreover, it has similar imaging characteristics to gastroenteropancreatic NET, which shows intense arterial enhancement and larger tumors often demonstrate cystic and necrotic portions (8). Thus, we suggest that presacral NET manifests as a well-enhancing mass with or without internal degeneration on MRI, reflecting its hypervascular nature.
MRI may also help to identify the relationship with the adjacent bone structure. Sacral destruction or remodeling was not seen in the two previously reported cases, and in one case pressure erosion of the coccyx was described (467). Our MRI showed loss of the normal fat plane between the mass and the sacrum and resultant pressure erosion. These imaging features were correlated with surgical findings which show that the mass was hardly fixed to the sacrum. Preoperative identification of the relationship of the tumor with the surrounding structures can allow the surgeon to plan the approach to the tumor. Moreover, MRI may help to predict the aggressiveness of the tumor preoperatively. Lower-grade pancreatic NET usually has a well-defined margin, whereas higher-grade NET tends to have an ill-defined margin due to its aggressive biology (9). Although correlation of MRI findings with histologic grading of presacral NETs is difficult due to its rarity, higher-grade presacral NETs may also have a tendency for infiltrative and irregular appearances with bony erosion, based on previous case reports and our case (67). In addition, we acquired DWI, in which the mass was hyperintense at a high b-value DWI with low ADC values, suggestive of tumor hypercellularity. However, this DWI finding might not aid in the differentiation of presacral NET from other presacral hypercellular solid tumors. The ADC values of NET may vary widely, possibly because of varying combinations of tumor cellularity, the proportion of cytoplasm, and extracellular fibrosis (9).
Radiologically, the differential diagnosis of presacral NET may include paraganglioma, solitary fibrous tumor and extraintestinal gastrointestinal stromal tumor (GIST), because these diseases usually manifest as hypervascular, predominantly solid presacral masses, and they less commonly remodel or destroy the sacrum (3). Paraganglioma of the retroperitoneum usually occurs in the organ of Zuckerkandl, and it is usually seen at the origin of the inferior mesenteric artery. On MRI, paraganglioma is typically a well-circumscribed, intensely enhancing mass with hyperintensity on T2-weighted images. Flow voids, hemorrhage, and the cap sign (a hypointense rim associated with hemorrhage) are commonly observed. Extraintestinal GIST in the presacral space is a rare entity, and it often contains regions of necrosis, hemorrhage, or cystic degeneration and tends to be aggressive. Extension into the ischiorectal fossa, prostate, or vagina may be present. Solitary fibrous tumor may occur anywhere in the body, and it typically appears as a well-circumscribed solid mass with intense heterogeneous enhancement. As a result of the fibrous tissue content, it has low to intermediate signal intensity on T1- and T2-weighted images. Flow voids and calcification are common in solitary fibrous tumors. However, due to some overlap in imaging features of hypervascular presacral tumors, pathologic examination is needed for making the final diagnosis.
In summary, we report a rare case of primary presacral NET. Despite its rarity, presacral NET could be included in the differential diagnosis of presacral hypervascular masses. MRI is the preferred modality for preoperative evaluation of presacral NET, and it helps the surgeon to determine a surgical plan.
Figures and Tables
Fig. 1
A 70-year-old woman presented with primary presacral neuroendocrine tumor.
A. Contrast-enhanced CT reveals an 8 cm-sized presacral mass (arrow) which abutted the sacrum without bony erosion or destruction. The mass is well-defined, solid with heterogeneous enhancement and it shows internal cystic degeneration and calcification.
B. Sagittal T2-weighted image (left) shows a presacral mass with pressure erosion and bone marrow change (arrow) in the sacrum. The mass shows heterogeneous intermediate signal intensity on T2-weighted image (left), hypointensity on T1-weighted image (middle), and avid enhancement on contrast-enhanced T1-weighted image (right). Focal hyperintense foci (arrowheads) within the mass are noted on T1- and T2-weighted images.
C. The tumor exhibits hyperintensity on diffusion-weighted image (left) at a b value of 1000 s/mm2 with a low apparent diffusion coefficient (ADC) value (mean, 0.632 × 10−3 mm2/sec) on the ADC map (right).
D. Photomicrograph of the tumor cells (left, hematoxylin and eosin stain, × 200) shows an infiltrative border in the background of fibrotic stroma. Tumor shows monomorphic, plasmacytoid neuroendocrine cells, with insular, solid and trabecular growth patterns. Immunohistochemical examinations show approximately 15% increase in the Ki-67 labeling index (middle, × 400) and positivity for synaptophysin (right, × 200).
E. Positron emission tomography-CT shows multiple pulmonary nodules, which are suspicious for metastasis.
Acknowledgments
This work was supported by clinical research grant from Pusan National University Hospital (2017).
References
1. Dujardin F, Beaussart P, de Muret A, Rosset P, Waynberger E, Mulleman D, et al. Primary neuroendocrine tumor of the sacrum: case report and review of the literature. Skeletal Radiol. 2009; 38:819–823.
2. Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010; 39:707–712.
3. Hain KS, Pickhardt PJ, Lubner MG, Menias CO, Bhalla S. Presacral masses: multimodality imaging of a multidisciplinary space. Radiographics. 2013; 33:1145–1167.
4. Lee JE, Shin KS, Cho JS, Kang DY, Kim JY. Presacral primary well differentiated neuroendocrine carcinoma: case report. J Korean Soc Radiol. 2011; 64:475–479.
5. Kim T, Grobmyer SR, Liu C, Hochwald SN. Primary presacral neuroendocrine tumor associated with imperforate anus. World J Surg Oncol. 2007; 5:115.
6. Misawa S, Horie H, Yamaguchi T, Kobayashi S, Kumano H, Lefor AT, et al. A unique retrorectal tumor with neuroendocrine differentiation: case report and review of the literature. Int J Surg Pathol. 2013; 21:271–277.
7. Wong JFS, Teo CCM. An unusual case of presacral carcinoid tumor and the approach of management. Am J Cancer Case Rep. 2013; 1:21–26.
8. Sahani DV, Bonaffini PA, Fernández-Del Castillo C, Blake MA. Gastroenteropancreatic neuroendocrine tumors: role of imaging in diagnosis and management. Radiology. 2013; 266:38–61.
9. Kim JH, Eun HW, Kim YJ, Han JK, Choi BI. Staging accuracy of MR for pancreatic neuroendocrine tumor and imaging findings according to the tumor grade. Abdom Imaging. 2013; 38:1106–1114.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download