Abstract
Granulomatosis with polyangiitis (GPA) is a multisystemic disease characterized by necrotizing granulomatous vasculitis, which histopathologically presents as vasculitis and granulomas with multinucleated giant cells. In pulmonary GPA, pulmonary cavity is a commonly observed imaging pattern although nodule and consolidation are the most common imaging findings. This cavitation follows a fluid-filled space within a pulmonary consolidation, a mass, or a nodule through the expulsion or drainage of a low-attenuation necrotic part of the lesion via the bronchial tree. However, in previous CT studies of pulmonary GPA, most cavitations appear as gas-filled spaces rather than accompanying necrotic fluid-attenuation areas. Therefore, we report a case of pulmonary GPA that presented with consolidations and nodules accompanied by a central low-attenuation area on CT, which mimicked septic pneumonia.
Granulomatosis with polyangiitis (GPA) is a multisystemic disease characterized by necrotizing granulomatous vasculitis, which histopathologically presents as vasculitis and granulomas with multinucleated giant cells. In the pulmonary manifestation of GPA, the CT findings are highly varied and they include nodules, consolidation, ground-glass opacity, and cavitation. Although pulmonary GPA is difficult to diagnose by imaging alone, pulmonary cavities and hemorrhage are the most common findings on a chest radiograph and CT scan. Approximately 6–50% of patients with pulmonary GPA have been reported to show cavitation on CT, and the percentage varies widely (12). In pulmonary GPA, previous CT studies have shown that most cavitations appear as gas-filled spaces with smooth and thin or irregular and thick walls rather than accompanying necrotic fluid-attenuation areas. Furthermore, most previous studies have focused on cavitation size, distribution, and number rather than the presence of necrotic fluid-attenuation foci in the nodules. Therefore, we report a case of pulmonary GPA that presented with consolidations and nodules accompanied by a central low-attenuation area on CT, which mimicked septic pneumonia at initial diagnosis.
A 31-year-old woman presenting with cough, sputum production, and fever up to 39℃ for a week was admitted to the emergency center at our tertiary institution. She had been diagnosed with acute otitis media 1 month ago and had received treatment with oral antibiotics and tympanostomy tube insertion at a primary care clinic. She had also been diagnosed with episcleritis 1 month ago, for which she had commenced treatment with steroid eye drops 3 days before admission. However, she had no other pulmonary symptoms such as hemoptysis, chest pain, or dyspnea, and she had no history of overseas travel, exposure to organic or inorganic dust, or immunosuppressive therapy. On physical examination, she presented coarse breath sounds, absence of crackles in both lungs, continuous discharge of pus from the middle ear cavity via the tube, and hyperemic conjunctiva in the left eye. Her laboratory data showed that the white blood cell count and the C-reactive protein level had increased to 17450/µL and 231 mg/L, respectively. On the initial chest radiograph, multifocal subsegmental consolidations were seen in both upper and mid lung zones, which were non-specific but were suggestive of pulmonary infection (Fig. 1A). On lung window CT images of subsequent chest CT scans, multiple nodules, ranging from 0.5 to 1.7 cm in size, and multiple subsegmental or lobular consolidations surrounded by ground-glass opacity were seen in both upper and lower lobes (Fig. 1B). On mediastinal window CT images of chest CT scans, central low-attenuation portions (presumably necrotic changes) were seen within the nodules and consolidations, which were suggestive of chronic necrotizing pneumonia or septic pneumonia resulting from a middle ear cavity infection (Fig. 1C). There was no evidence of pulmonary thromboembolism, mediastinal or hilar lymphadenopathy, and pleural or pericardial effusion. Echocardiography performed to exclude the possibility of infective endocarditis showed no microbial vegetation, but it showed prolapse of the tricuspid valve. One day after treatment with empirical antibiotics, her cough and fever aggravated; moreover, the consolidations increased in their extent and were surrounded by ground-glass opacity even on follow-up chest radiography. Polymerase chain reaction analysis of the sputum revealed the absence of Mycobacterium tuberculosis. The sputum and blood cultures tested negative for microorganisms including fungi, bacteria and viruses. Considering the refractoriness to antibiotics and multisystemic involvement of the ear, eye, and lung, the possibility of granulomatous vasculitis such as GPA was suggested. Autoimmune antibody sampling and percutaneous core needle lung biopsy were performed for confirming the diagnosis. Histopathologic analysis of the lung biopsy specimen performed on the 4th hospital day revealed necrosis with granulomas including multinucleated giant cells, which were consistent with the findings of GPA (Fig. 1D). On the 5th hospital day, the consolidations increased in their extent and were surrounded by ground-glass opacity even on the follow-up chest radiograph (Fig. 1E). The pulmonologist decided to administer immunosuppressive agents such as steroids and cyclophosphamide. Four days after initiating the immunosuppressive therapy, her fever, cough, and sputum production improved, as did her pyogenic otorrhea and hyperemic conjunctiva. Autoimmune antibody sampling showed an increased PR3-ANCA antibody index of 8.70 and the absence of MPO-ANCA, and both of these findings corresponded to the diagnosis of GPA. On the 8th day of immunosuppressive therapy, her fever completely subsided and the consolidation with surrounding ground-glass opacity decreased in its extent and changed into irregular thick walled cavitary lesions on the follow-up chest radiograph (Fig. 1F), and she was discharged. However, seven days after discharge, she experienced a recurrence of fever up to 38℃, but the consolidations had decreased and changed to air-filled, thick-walled, cavitary lesions, as revealed by the follow-up chest radiograph. She was treated with an increased dose of steroid and cyclophosphamide because of persistent fever up to 38℃. Thereafter, follow-up chest radiography performed 20 days after discharge revealed that the consolidations had markedly decreased in their extent and the cavity wall was much thinner.
In patients with pulmonary GPA, nodules and masses were the most common radiologic findings (i.e., in 50–90% of the patients), and accompanying cavitation was present in 15–22% of the nodules (12). However, in contrast to cavitation, necrosis within the nodules or masses was less commonly reported in previous studies. Guneyli et al. (1) reported that bilateral or unilateral necrosis was observed in 16% of all nodules and masses. Necrosis within the nodules or masses appears as bilateral or unilateral, central low attenuations with or without peripheral enhancement on mediastinal window images of enhanced CT scans; this finding reflects extensive geographic necrosis with a tendency for cavitation observed on histopathological analysis. Differential diagnoses of multiple masses with central low-attenuated necrosis include necrotizing pneumonia, pulmonary infarction, metastasis, and lung abscess. However, linear scarring, spiculation, and pleural tags are helpful in differentiating these diseases from pulmonary GPA, but these findings were not observed in our case (1). In our case, we think that central necrosis with low attenuation was accompanied by multiple nodules and wedge-shaped consolidations on CT because CT scanning was performed before the expulsion of necrotic materials from the nodules.
Another radiologic finding of pulmonary GPA is ground-glass opacity surrounding the nodules or consolidations, i.e., the halo sign, which results from either alveolar hemorrhage or intraalveolar cellular debris. The halo sign is considered the result of adjacent parenchymal hemorrhage seen in 15% of the cases (2). However, this halo sign may also occur in cancer, metastasis, and angioinvasive infections, such as Aspergillus fumigatus infections, as well as pulmonary GPA. In our case also, the halo sign, presumably caused by adjacent parenchymal hemorrhage, was seen in the right upper and middle lobes on initial chest CT, although the surrounding ground-glass opacity was not detected on initial chest radiography. These ground-glass opacities started appearing around the consolidations in both upper lobes on chest radiography performed on the 3rd hospital day during disease progression. After 6 days of immunosuppressive therapy, these opacities disappeared with a decrease in the consolidations.
In patients with GPA, the upper respiratory tract was commonly involved, and sinusitis, otitis media, polychondritis, vertigo, and sudden hearing loss also occurred (3). Ocular involvement in GPA results in conjunctivitis, corneal ulceration, episcleritis, scleritis, optic neuropathy, retinal vasculitis, and uveitis, which develop in up to 58% of the patients before or after systemic manifestation (4). Otologic manifestation is also a common initial symptom in 73% of the patients (5). In particular, the prognosis of the generalized form of GPA resulting from otologic GPA is worse than that of the limited form of GPA, because the generalized form more often indicates pulmonary insufficiency or renal failure (6). In our case, the patient had refractory otitis media and episcleritis, left eye, which are the otologic and ocular manifestations of GPA, before she developed the pulmonary manifestation of GPA. However, the relationship between the pulmonary manifestation and the other systemic manifestations of GPA is still unclear.
Induction therapy for GPA includes the administration of cyclophosphamide or rituximab, with or without a glucocorticoid, or the administration of a glucocorticoid alone. In our case, a combination of cyclophosphamide and glucocorticoid was adopted as the initial therapy. Hoffman et al. (7) reported that this combination therapy induced marked symptom relief in 91% of the patients and complete remission in 75% of the patients. In our case, the patient's pulmonary symptoms and radiographic findings improved within 2 weeks of initiating the combination therapy. Although the response to treatment is good, relapse is common in GPA, and approximately 50% of the patients experience a relapse (8). Farrelly (9) reported that a relapse of GPA more commonly involved the previously damaged area. Although the relationship between relapse and prognosis is still controversial, a poor prognosis is associated with older age and damage to target organs such as in cases of pulmonary manifestation, cardiac manifestation, renal manifestation, and chronic nasal carriage of Staphylococcus aureus (10).
In conclusion, we reported a case of pulmonary GPA presenting with multiple consolidations and nodules accompanied by a central low-attenuation area on CT, which mimicked septic pneumonia resulting from middle ear infection at initial diagnosis. Although cavitation is a more common CT feature of pulmonary GPA nodules or masses than is low attenuation of necrosis, this cavitation may be detected uncommonly when CT is performed before the expulsion of necrotic materials from the nodules. Therefore, the possibility of pulmonary GPA should be taken into consideration when multiple nodules or consolidations are accompanied by central low-attenuation areas in patients with upper respiratory infection, other systemic manifestations or no response to antibiotic therapy.
Figures and Tables
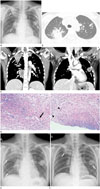
Fig. 1
A 31-year-old woman with pulmonary granulomatosis with polyangiitis mimicking septic pneumonia resulting from middle ear infection.
A. Chest radiograph shows multifocal consolidations in both upper and mid lung zones (arrows).
B. Axial lung window image of high-resolution computed tomography shows subsegmental and lobular consolidations surrounded by ground-glass opacity in both upper lobes (arrows).
C. Coronal mediastinal window images of contrast enhanced chest CT scan show central low-attenuation portions (presumably necrotic changes) within the consolidations in both upper and right lower lobes (arrows).
D. Photomicrographs of the biopsy specimen show severe necrotizing inflammation around the vessels (arrow) and granulomatous inflammation with multinucleated giant cells (arrowheads) (hematoxylin and eosin, × 200).
E. Chest radiograph obtained on the 5th hospital day shows that the consolidations have increased in their extent in both lungs and are surrounded by ground-glass opacity in the right lung (arrows).
F. Chest radiograph taken 8 days after immunosuppressive therapy reveals that the consolidation with surrounding ground-glass opacity has decreased in its extent and changed into irregular thick walled cavitary lesions in both lungs (arrows).
References
1. Guneyli S, Ceylan N, Bayraktaroglu S, Gucenmez S, Aksu K, Kocacelebi K, et al. Imaging findings of pulmonary granulomatosis with polyangiitis (Wegener's granulomatosis): lesions invading the pulmonary fissure, pleura or diaphragm mimicking malignancy. Wien Klin Wochenschr. 2016; 128:809–815.
2. Lee KS, Kim TS, Fujimoto K, Moriya H, Watanabe H, Tateishi U, et al. Thoracic manifestation of Wegener's granulomatosis: CT findings in 30 patients. Eur Radiol. 2003; 13:43–51.
3. Seo P, Stone JH. The antineutrophil cytoplasmic antibody-associated vasculitides. Am J Med. 2004; 117:39–50.
4. Harper SL, Letko E, Samson CM, Zafirakis P, Sangwan V, Nguyen Q, et al. Wegener's granulomatosis: the relationship between ocular and systemic disease. J Rheumatol. 2001; 28:1025–1032.
5. Erickson VR, Hwang PH. Wegener’s granulomatosis: current trends in diagnosis and management. Curr Opin Otolaryngol Head Neck Surg. 2007; 15:170–176.
6. Wierzbicka M, Szyfter W, Puszczewicz M, Borucki L, Bartochowska A. Otologic symptoms as initial manifestation of wegener granulomatosis: diagnostic dilemma. Otol Neurotol. 2011; 32:996–1000.
7. Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992; 116:488–498.
8. Renaudineau Y, Le Meur Y. Renal involvement in Wegener's granulomatosis. Clin Rev Allergy Immunol. 2008; 35:22–29.
9. Farrelly CA. Wegener’s granulomatosis: a radiological review of the pulmonary manifestations at initial presentation and during relapse. Clin Radiol. 1982; 33:545–551.
10. Mukhtyar C, Flossmann O, Hellmich B, Bacon P, Cid M, Cohen-Tervaert JW, et al. Outcomes from studies of antineutrophil cytoplasm antibody associated vasculitis: a systematic review by the European League Against Rheumatism systemic vasculitis task force. Ann Rheum Dis. 2008; 67:1004–1010.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download