Abstract
Extra-adrenal paraganglioma is a rare tumor arising from the neural crest cells. Most tumors that develop in the abdomen arise from paraganglia along the paravertebral and para-aortic areas, in particular the organ of Zuckerkandl, which is close to the origin of the inferior mesenteric artery. However, extra-adrenal paraganglioma also occurs in relatively rare places such as the urinary bladder, gallbladder, hepatoduodenal ligament, and gastrointestinal tract. Here, we report imaging findings of extra-adrenal paragangliomas presenting as mesenteric and pararectal masses with lymph node metastasis.
Paraganglioma is a family of tumors originating from the neural crest cells. Tumor occuring within the adrenal medulla are called pheochromocytoma, and those developing in extra-adrenal locations (5–10% of cases) are referred to as paraganglioma (12). Paraganglioma secrete catecholamines and cause malignant hypertension or symptoms such as headaches, palpitations, and diaphoresis. They can be located at any anatomical site along the paravertebral and para-aortic areas, from the head to the pelvic floor. Extra-adrenal paraganglioma that develops within the abdomen are distributed along the paravertebral and para-aortic axis, in particular, along the organ of Zuckerkandl (close to the origin of the inferior mesenteric artery). However, several studies have reported the occurrence of extra-adrenal paraganglioma in relatively rare locations, such as the urinary bladder, gallbladder, and hepatoduodenal ligament (345). Paraganglioma located in the mesentery or pararectal space is extremely rare, and only a few reports of such masses have been published (126789).
We report here an unusual case of malignant, extra-adrenal paragangliomas, presenting as mesenteric and pararectal masses with lymph node metastasis. This paper includes their imaging features and histopathology characteristics.
A 70-year-old man visited our institution for evaluation and management of abdominal masses incidentally detected at a local clinic. The patient had no specific symptoms or relevant past medical history. All laboratory findings, including plasma catecholamine levels, were within the normal range. The patient had undergone computed tomography (CT) scans at an outside hospital to evaluate the intra-abdominal masses using a 16-slice multidetector CT scanner (SOMATOM Emotion 16; Siemens, Erlangen, Germany). Contrast-enhanced axial CT images revealed a multilobulated, solid mass, approximately 15 cm in size, showing heterogeneous and strong enhancement, with adjacent engorged vessels in the small bowel mesentery (Fig. 1A). A second round mass at the right side of the rectum, 2.8-cm in size, well defined, and with a similar enhancement pattern, was observed on the CT scan (Fig. 1B). The patient further underwent magnetic resonance (MR) imaging to assess the exact anatomic location, using a 3T unit (MAGNETOM Skyra, Siemens, Erlangen, Germany) with a standard 18-channel body matrix coil and table-mounted 32-channel spine matrix coil. For the enhancement studies, gadoxetic acid as a contrast agent (Gd-EOB-DTPA, Primovist; Bayer Healthcare, Berlin, Germany) was administered intravenously. MR imaging revealed well-defined solid masses in the small bowel mesentery and pararectal area, showing heterogeneous hypo- and hyper-signal intensities on both T1- (Fig. 1C) and T2-weighted images (Fig. 1D). On dynamic enhancement, the tumors showed avid, heterogeneous enhancement with poor central enhancement that matched the high signal intensity on the T2-weighted image. On the diffusion-weighted MR image with a b-value of 800 s/mm2, the mesenteric mass showed high signal intensity, and the peripheral portion of the mass had a low value on the apparent diffusion coefficient (ADC) map, indicating the presence of restricted diffusion (Fig. 1E). No evidence of restricted diffusion in the pararectal mass was seen.
Differential diagnoses based on these imaging findings included a gastrointestinal stromal tumor, neuroendocrine tumor, paraganglioma, and hypervascular metastasis. A subsequent exploratory laparotomy revealed a large mass with hemorrhage, originating from the small bowel mesentery, and another mass in the right pararectal area. Since the pararectal mass had invaded the rectal wall, a low anterior resection was performed. The histopathological examination revealed cellular neoplasms composed of uniform small round cells separated by fibrovascular connective tissue, showing a characteristic zellballen pattern (Fig. 1F). The mitotic count was low (< 1/10 high power field). Immunohistochemically, the tumor cells were positive for synaptophysin and chromogranin. The proportion of Ki-67 positive cells was 10%.
On the basis of the histopathologic findings, the masses in the small bowel mesentery and pararectal area were confirmed as paragangliomas. Metastasis was identified in 1 out of the 18 resected mesenteric lymph nodes, and the masses were finally diagnosed as malignant paragangliomas. The postoperative course was uneventful, and the patient was discharged on postoperative day 8.
Paraganglioma is a rare tumor derived from neural crest cells that develop into the sympathetic and parasympathetic paraganglia. When it secretes catecholamines, it is called a functioning paraganglioma. The most common clinical symptoms associated with catecholamine secretion include headaches, palpitations, and profuse sweating. From a diagnostic viewpoint, a functional tumor is easier to diagnose. However, similar to our case, most extra-adrenal paragangliomas are nonfunctional (9). Our patient was devoid of any clinical symptoms or abnormal laboratory findings. Therefore, the clinical presentation and biochemical markers were non-informative in making the diagnosis. In these situations, imaging studies play an important role in characterizing the tumors and determining their location.
Extra-adrenal paraganglioma are usually observed as welldefined, round- or oval-shaped masses of soft-tissue density on unenhanced CT scans. Punctate parenchymal calcification is observed in about 15% of such tumors. As the tumor progresses, it often appears cystic with central necrosis. Intense contrast enhancement is observed due to the hypervascular nature of paragangliomas. On MR imaging, extra-adrenal paraganglioma is usually hypointense or isointense on T1-weighted images, and hyperintense on T2-weighted images. Heterogeneous signal intensity is often seen, owing to the presence of hemorrhage. In addition, a speckled appearance with multiple flow voids is typical. Imaging findings of extra-adrenal paraganglioma on diffusion-weighted MR imaging and ADC map have been described in very few reports (3). Extra-adrenal paraganglioma shows high signal intensity on diffusion-weighted MR imaging with high b-values. It displays a low value on the ADC map, indicative of restricted diffusion. In our patient, the mesenteric and pararectal paragangliomas showed high signal intensity on diffusion-weighted MR imaging with a high b-value gradient. However, restricted diffusion was seen only in the peripheral portions of the mesenteric paraganglioma.
Despite positive laboratory test results, if a lesion is not identified on radiologic studies, radionuclide imaging with 123I-metaiodobenzylguanidine or 18F-fluorodeoxyglucose positron emission tomography is recommended to identify the occult lesion. Radionuclide imaging is a more practical way of examining the entire body, and is valuable in localizing a functional extra-adrenal paraganglioma and its associated metastases.
The gross morphology of a paraganglioma is usually sharply circumscribed with expanding borders; on observing the cross section, this tumor has resiliently firm and grey-white surface, with areas of hemorrhage and cystic degeneration within the tumor. Microscopically, the most characteristic pattern is an organoid or trabecular arrangement (zellballen) of neoplastic chief cells, separated by a rich microvasculature. Intracytoplasmic granules, similar to those seen in healthy paraganglia, can be seen in some tumors. Although prominent nuclear hyperchromasia and a remarkable degree of nuclear pleomorphism may be present, these are not reliable features in diagnosing malignancy. Benign and malignant paragangliomas have an identical histologic appearance, and the criteria for malignancy on the basis of histopathology are not well defined. Therefore, local invasion or distant metastasis are important features to be considered in the diagnosis of a malignant paraganglioma. Approximately 2–36% of paragangliomas are diagnosed as malignant, based on extensive local invasion or metastasis (10). In general, a paraganglioma is more likely to be malignant than a pheochromocytoma.
Although most paragangliomas are solitary and arise sporadically, they can be multicentric and can occur synchronously or metachronously; the incidence rate of multicentricity is approximately 10% (10). Our case showed 2 extra-adrenal paragangliomas in the small bowel mesentery and the pararectal area. The smaller paraganglioma in the pararectal area may have been a synchronous tumor or metastases from the mesenteric paraganglioma. Either way, the mesenteric paraganglioma was identified as malignant due to the presence of mesenteric lymph node metastasis.
The differential diagnosis for such hypervascular masses includes solitary fibrous tumors, gastrointestinal stromal tumors, and hypervascular metastases of carcinoma and melanoma. Considering the hypervascular nature of the tumors in this case, extra-adrenal paraganglioma was included in the differential diagnosis. However, its distant location from the para-aortic area and the rarity of mesenteric and pararectal paragangliomas, made a preoperative diagnosis difficult.
We conclude by summarizing that extra-adrenal paraganglioma in the abdomen is rarely found in the mesenteric or pararectal location. It can manifest as a heterogeneous, hypervascular solid mass that should be differentiated from a mesenchymal tumor or metastases. Although rare, extra-adrenal paraganglioma should be included in the preoperative differential diagnosis of a solid hypervascular intra-abdominal mass, regardless of the tumor location.
Figures and Tables
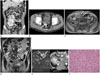 | Fig. 1Mesenteric and pararectal paragangliomas in a 70-year-old man.
A. The portal venous phase coronal computed tomography (CT) scan shows a 15 cm-sized, heterogeneous, hypervascular soft tissue mass, with a lobulated contour (arrows) in the small bowel mesentery of the abdomen.
B. The contrast enhanced portal venous phase axial CT image shows another well-defined, hypervascular mass, approximately 2.8 cm in size, adjacent to the right rectal wall (arrow).
C. The T1-weighted axial magnetic resonance (MR) image without a contrast agent shows the heterogeneous signal intensity of the mesenteric mass (arrows), which is similar to that of the pararectal mass (not shown).
D. The T2-weighted coronal MR image reveals a large, lobulated soft tissue mass (arrows) with heterogeneous high signal intensity, internal signal void structures, and engorged supplying vessels (blank arrows) in the small bowel mesentery. Heterogeneous high signal intensity with internal signal void was observed in the pararectal mass (not shown).
E. The high signal intensity of the mesenteric mass (arrows) is shown on the diffusion-weighted MR image with a b-value of 800 s/mm2 (left). Some portions of the mesenteric mass show a low apparent diffusion coefficient value (arrows), indicating the presence of diffusion restriction (right).
F. Microscopic features of the paraganglioma. Some tumor cells show a nested pattern, and others show a diffuse pattern. The tumor cells exhibit eosinophilic cytoplasm and ‘salt and pepper’ patterned chromatin with moderate nuclear pleomorphism (hematoxylin-eosin stain; original magnification, × 200), consistent with the findings of a paraganglioma.
|
References
1. Fujita T, Kamiya K, Takahashi Y, Miyazaki S, Iino I, Kikuchi H, et al. Mesenteric paraganglioma: report of a case. World J Gastrointest Surg. 2013; 5:62–67.
2. Ozkan Z, San Ozdemir C, Yasar G, Altas O, Koc M, Gul Y, et al. An unusual mesenteric tumor ‘paraganglioma’: a case report. Iran Red Crescent Med J. 2014; 16:e16837.
3. Wang H, Ye H, Guo A, Wei Z, Zhang X, Zhong Y, et al. Bladder paraganglioma in adults: MR appearance in four patients. Eur J Radiol. 2011; 80:e217–e220.
4. Baker C, Bhagwat P, Wan A. Mesenteric paraganglioma with gallbladder paraganglion nest. J Surg Case Rep. 2012; 2012:8.
5. Furukawa K, Ishida H, Komatsuda T, Yagisawa H, Yamada M, Miyauchi T, et al. Malignant paraganglioma draining into the main portal vein. Abdom Imaging. 2005; 30:758–760.
6. Chetrit M, Dubé P, Royal V, Leblanc G, Sideris L. Malignant paraganglioma of the mesentery: a case report and review of literature. World J Surg Oncol. 2012; 10:46.
7. Kudoh A, Tokuhisa Y, Morita K, Hiraki S, Fukuda S, Eguchi N, et al. Mesenteric paraganglioma: report of a case. Surg Today. 2005; 35:594–597.
8. Moslemi MK, Abolhasani M, Vafaeimanesh J. Malignant abdominal paraganglioma presenting as a giant intra-peritoneal mass. Int J Surg Case Rep. 2012; 3:537–540.
9. Bhatt S, Vanderlinde S, Farag R, Dogra VS. Pararectal paraganglioma. Br J Radiol. 2007; 80:e253–e256.
10. Lee KY, Oh YW, Noh HJ, Lee YJ, Yong HS, Kang EY, et al. Extraadrenal paragangliomas of the body: imaging features. AJR Am J Roentgenol. 2006; 187:492–504.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download