Abstract
Purpose
The purpose of this study is to evaluate the clinical safety and usefulness of the Prosure®300 in contrast-enhanced abdominal CT.
Materials and Methods
This prospective study was approved by our center's Institutional Review Board. This study included 727 patients in four hospitals who underwent contrast-enhanced abdominal CT using Prosure®300 from December 2010 to June 2011. Adverse events were classified into minor and major adverse events. Logistic regression analysis was used to evaluate the relationship between adverse events and patient gender, age, underlying disease, and amount of injected contrast agent. Two radiologists independently evaluated imaging quality as poor, insufficient, sufficient, good, or very good.
Results
One hundred seventy-six out of 727 patients complained of adverse events, but most of them were minor adverse events. Five patients complained of dyspnea and one patient had hoarseness, but recovered without treatment. The rate of adverse events was significantly higher in men (p = 0.011), and a greater amount of injected contrast agent was related to a higher rate of adverse events (p = 0.000). Imaging quality was evaluated as ‘good’ or ‘very good’ in all cases.
Figures and Tables
Fig. 1
Molecular structure of iopromide. There are 4 of hydroxyl groups, which confer hydrophilicity.
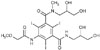
Fig. 2
Axial scan of the arterial phase (A, B) of an abdominal CT scan performed with Prosure®300. The overall image quality and enhancement of the superior mesenteric artery, liver, kidneys, and pancreas in (A) were good. Decreased image quality and enhancement of liver, kidneys and pancreas in (B) are present due to a motion artifact. The image quality of (A) was evaluated as ‘very good,’ while that of (B) was evaluated as ‘good.’
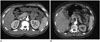
Table 1
Incidences of Adverse Events (AEs)

Table 2
Complication Rates in Patients Trouped According to Each Variables and the Result of Logistic Regression Analysis

References
1. Lee JT, Suh JH, Suh JS, Lee YH. Clinical application and side effects of non-ionic, low-osmolar contrast media: iopromide (Ultavist). J Korean Soc Radiol. 1988; 24:324–329.
2. Grainger RG. Osmolality of intravascular radiological contrast media. Br J Radiol. 1980; 53:739–746.
3. Numaguchi Y, Fleming MS, Hasuo K, Puyau FA, Nice CM Jr. Blood-brain barrier disruption due to cerebral arteriography: CT findings. J Comput Assist Tomogr. 1984; 8:936–939.
4. Hilal SK, Dauth GW, Hess KH, Gilman S. Development and evaluation of a new water-soluble iodinated myelographic contrast medium with markedly reduced convulsive effects. Radiology. 1978; 126:417–422.
5. Sacks BA, Ellison HP, Bartek S, Vine HS, Palestrant AM. A comparison of hexabrix and Renografin 60 in peripheral arteriography. AJR Am J Roentgenol. 1983; 140:975–977.
6. Castel JC, Dorcier F, Caillé JM. Penetration of the brain by nonionic water soluble tri- and hexaiodinated contrast media. Experimental autoradiographic study of two contrast media: Iotrol and Iopamidol labelled with iodine 125. Neuroradiology. 1987; 29:206–210.
7. Hong HS, Kim DH, Lee HK, Jung MC, Choi DL, Kwon KH, et al. Clinical application of intravascular administration of non-ionic, low osmolar contrast agent, ioversol and its side effects comparison with meglumine iothalamate. J Korean Soc Radiol. 1990; 26:1284–1290.
8. Rapoport SI, Levitan H. Neurotoxicity of X-ray contrast media. Relation to lipid solubility and blood-brain barrier permeability. Am J Roentgenol Radium Ther Nucl Med. 1974; 122:186–193.
9. Ing JJ, Smith DC, Bull BS. Differing mechanisms of clotting inhibition by ionic and nonionic contrast agents. Radiology. 1989; 172:345–348.
10. Bae K, Lee SM, Ha JY, Jeon KN, Moon JI, Choi BH, et al. Adverse drug reactions to CT contrast media in South Korea: incidence and risk factors. J Korean Soc Radiol. 2016; 75:41–48.
11. Grainger RG. Intravascular contrast media. Br J Radiol. 1982; 55:544.
12. McClennan BL, Stolberg HO. Intravascular contrast media. Ionic versus nonionic: current status. Radiol Clin North Am. 1991; 29:437–454.
13. Stolberg HO, McClennan BL. Ionic versus nonionic contrast use. Curr Probl Diagn Radiol. 1991; 20:47–88.
14. Wolf GL, Arenson RL, Cross AP. A prospective trial of ionic vs nonionic contrast agents in routine clinical practice: comparison of adverse effects. AJR Am J Roentgenol. 1989; 152:939–944.
15. Kennedy C, Rickards D, Lee S, Sharp MB, Dawson P. A double-blind study comparing the efficiency, tolerance and renal effects of iopromide and iopamidol. Br J Radiol. 1988; 61:288–293.
16. Vlahos L, Dimakakos P, Moulopoulou A, Drivas G, Keysser R, Papavasiliou C. Comparative study between iohexol and iopromide for aortofemoral arteriography. Radiologe. 1987; 27:581–582.
17. Dyet JF, Carter EC, Hartley WC. Comparison of iopromide and iopamidol in left ventricular angiography and in coronary angiography. Br J Radiol. 1990; 63:700–705.
18. Haughton VM, Papke RA, Hyland D, Drayer BP, Osborn AG, Maravilla K, et al. Safety and efficacy of iopromide in cerebral arteriography. Invest Radiol. 1994; 29:Suppl 1. S94–S97.
19. Goldberg SN, Abrahams J, Drayer BP, Golding S, Bernardino M, Brunetti J. A comparison of iopromide with iopamidol and iohexol for contrast-enhanced computed tomography. Invest Radiol. 1994; 29:Suppl 1. S76–S83. discussion S93.
20. Newhouse JH, Landman J, Lang E, Amis ES, Goldman S, Khazan R, et al. Efficacy and safety of iopromide for excretory urography. Invest Radiol. 1994; 29:Suppl 1. S68–S73.
21. Lee GJ, Kim SH, Park JH, Chang KH, Han MC, Kim JW. Clinical trial of non-ionic contrast media. J Korean Soc Radiol. 1988; 24:349–357.
22. Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990; 175:621–628.
23. Mortelé KJ, Oliva MR, Ondategui S, Ros PR, Silverman SG. Universal use of nonionic iodinated contrast medium for CT: evaluation of safety in a large urban teaching hospital. AJR Am J Roentgenol. 2005; 184:31–34.
24. Kim MH, Seon HJ, Choi S, Kim YH, Kim JK, Park JG, et al. Clinical utility of iopamidol (Pamiray®370) for cardiac CT. J Korean Soc Radiol. 2011; 65:27–33.
25. Park SH, Suh SH, Kim J, Kim EY, Kim DJ, Lee SK, et al. Clinical application of iopamidol (Pamiray® 300) for cerebral angiography. J Korean Soc Radiol. 2007; 57:121–127.
26. Cochran ST, Bomyea K, Sayre JW. Trends in adverse events after IV administration of contrast media. AJR Am J Roentgenol. 2001; 176:1385–1388.
27. Velasco MA, Perez GE, Cortejoso HF. Monitoring of adverse reactions to iodinated contrast media in in-patients and out-patients. Farm Clin. 1996; 13:596–609.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download