Abstract
Myeloid sarcoma is an extramedullary solid neoplasm composed of myeloid precursor cells. This tumor usually occurs simultaneously with or following the onset of acute leukemia. Rarely, it can be the first manifestation of acute myeloid leukemia. The tumor can occur anywhere in the body. However, primary involvement of the stomach without evidence of leukemia is exceedingly rare, and to the best of our knowledge, imaging features of isolated myeloid sarcoma of the stomach have not been reported in children. This case illustrates the imaging appearances of isolated myeloid sarcoma that initially manifested as gastric submucosal wall thickening and discusses the differential diagnosis, in a 15-year-old girl without evidence of hematologic malignancy.
Myeloid sarcoma (MS), also known as chloroma, granulocytic sarcoma or extramedullary myeloblastoma, is a collection of myeloid precursor cells that include myeloblasts, promyelocytes, and myelocytes (12). MS usually occurs in the course of acute or chronic myeloid leukemia or leukemic transformation in myeloproliferative or myelodysplastic disorders. But it has rarely been reported as an isolated manifestation in non-leukemic patients (1). MS has been found in almost every anatomic location, and it more commonly occurs in the skin, bone, soft tissue, lymph nodes and central nervous system (CNS) (123). However, involvement of the gastrointestinal tract, especially the stomach, is uncommon, and radiologic findings of stomach involvement have been rarely reported in the literature (4). We report the imaging features, including those of ultrasonography, CT and positron emission tomography (PET)-CT, in an unusual case of isolated MS of the stomach in a 15-year-old girl without evidence of any hematologic disorder.
A 15-year-old girl was admitted to our hospital with intermittent epigastric pain for 3 months. She had no specific medical or surgical history. Physical examination revealed mild tenderness in the epigastric and left upper quadrant areas of the abdomen. There was no hepatosplenomegaly or lymphadenopathy. Laboratory evaluations showed a white blood cell count of 6300/mm3, a hemoglobin level of 10.9 g/dL, and a platelet count of 337000/mm3. Serum lactate dehydrogenase level was 498 IU/L, which was slightly elevated.
An abdominal ultrasonogram (GE LOGIQ 9; GE Healthcare, Milwaukee, WI, USA) showed marked hypoechoic wall thickening of the gastric body (Fig. 1A). Abdominal CT (LightSpeed VCT; GE Healthcare) revealed marked and eccentric wall thickening with homogeneous contrast enhancement in the stomach, particularly the lesser curvature of the body portion (Fig. 1B, C). Mucosa of the thickened gastric wall and perigastric fat planes were smooth and preserved. There was no perigastric or distant lymphadenopathy. 18F-fluorodeoxyglucose (FDG) PET-CT (GE Discovery 600; GE Healthcare) was performed for further staging because the presumptive diagnosis was a lymphoma. On the PET-CT images, increased 18F-FDG was found only in the lesser curvature side of the gastric body with a maximal standardized uptake value (SUV) of 15.2, suggestive of a malignant lesion (Fig. 1D). There was no other abnormal FDG uptake lesion. Upper GI endoscopy was performed for biopsies, which showed extensive enlarged and hyperemic fold thickening with scattered hemorrhagic spots in the lesser curvature of the gastric body (Fig. 1E). Histopathological findings from the endoscopic biopsy specimens were consistent with MS based on the presence of cells of the myeloid lineage with positive immunohistochemical staining for myeloperoxidase and myeloid markers (CD 34, CD 43, CD 117), and negative immunohistochemical staining for B-cell (CD 20) and T-cell markers (CD 3 and CD 30) (Fig. 2). Bone marrow examination demonstrated no evidence of marrow involvement with acute myeloid leukemia (AML) blasts.
The patient received cytarabine-based induction and consolidation chemotherapy. Unfortunately, 6 months later, the patient noted relapse in the stomach, breast and peritoneum and showed AML M4 type with a blast count of 33% on bone marrow examination. Second-line chemotherapy and radiation therapy were applied quickly, but the patient died due to disease dissemination and septic shock.
MS is a rare, malignant localized solid tumor composed of immature granulocytic precursor cells occurring in extramedullary sites. In the majority of cases, it is diagnosed at presentation or during the course of AML. The overall prevalence of MS was 2.5–8% in one autopsy series of acute leukemia (1). In a multicenter study of 1832 children with AML, 199 (10.9%) patients had MS and only 13 (0.7%) patients had isolated MS without involvement of the bone marrow (2). Another literature has reported that isolated MS without any blood or bone marrow involvement at the time of diagnosis is a rare disease with an incidence of 2/1000000 in adults (5). MS can occur virtually anywhere in the body; the most common sites are skin, orbit, head and neck, bone and CNS (13). MS involving the gastrointestinal tract is relatively rare and it occurs mostly in the small bowel (14). The involvement of the stomach is very rare, and to the best of our knowledge, the imaging features of isolated MS of the stomach have not been reported in children.
There are a few reports on the radiological features of MS in the gastrointestinal tract and the imaging features of MS are variable; lesions can appear as an intramural or exophytic polypoid mass or bowel wall thickening or a combination of these manifestations (46). In our case, the wall of the stomach was markedly thickened and showed homogeneous contrast enhancement. The gastric wall thickening was eccentric, and it was not circumferential. Overlying mucosa of the thickened gastric wall and perigastric fat planes were smooth on CT and gastric fold was preserved on gastroscopy, suggestive of submucosal disease involvement. There was no regional or distant lymphadenopathy. In 18F-FDG PET-CT images, the thickened gastric wall showed diffuse hypermetabolism with a maximal SUV of 15.2. These imaging features of the gastric lesion were quite similar to those of a primary gastric lymphoma and these features cannot be distinguished from those of a primary gastric lymphoma, although circumferential wall thickening and regional or mesenteric lymphadenopathy are frequently seen in the majority of lymphomas (7). Inflammatory myofibroblastic tumor and gastrointestinal stromal tumor (GIST) should also be included in the differential diagnosis because both gastric lesions may show a well-defined exophytic or submucosal mass (89). The gastric inflammatory myofibroblastic tumor shows various appearances, but it usually shows aggressive features such as ulceration, adjacent wall infiltration and extraluminal extension, which were not seen in this case (810). Gastric GIST is an uncommon mesenchymal tumor in adolescents and children and it appears as a submucosal mass of varying size with normal overlying mucosa. But, GIST usually shows central necrosis, cavitation, cyst formation, and hemorrhage, when it presents as a large tumor (9).
In an unusual manifestation like our case, in which the tumor precedes the hematologic evidence of leukemia, it may be impossible to diagnose MS of the stomach initially and it can lead to diagnostic challenges. This case illustrates the radiologic findings including those of ultrasonography, CT, and PET-CT of isolated MS that presented as eccentric and smooth wall thickening of the stomach without regional or distant lymphadenopathy.
Figures and Tables
Fig. 1
Multimodal imaging of isolated myeloid sarcoma in a nonleukemic child.
A. Oblique axial gray-scale ultrasonogram shows marked and hypoechoic thickening of the stomach wall (arrows) with loss of stratification.
B, C. Coronal reformatted, contrast-enhanced CT images demonstrate eccentric, marked and homogeneous wall thickening (asterisks) in the lesser curvature of the gastric body. Mucosa of the thickened gastric wall (thin arrows) and perigastric fat planes (thick arrows) are smooth and preserved, which are suggestive of submucosal disease involvement. There is no regional or distant lymphadenopathy.
D. Coronal 18F-FDG PET-CT image shows a diffuse hypermetabolic area (maximal standardized uptake value of 15.2) corresponding to the thickened gastric wall (asterisk).
E. Upper GI endoscopy reveals extensive enlarged and hyperemic fold thickening (asterisks) with scattered hemorrhagic spots.
GI = gastrointestinal, 18F-FDG PET-CT = 18F-fluorodeoxyglucose PET-CT
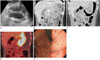
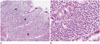
Fig. 2
Histopathologic examination of isolated myeloid sarcoma in a nonleukemic child.
A. Photomicrograph (hematoxylin-eosin stain, × 100 magnification) shows diffuse infiltrates of leukemic blasts and some maturing granulocytes (asterisks) in the submucosal tissue and intact overlying mucosa (M) of the stomach.
B. Microscopic image (hematoxylin-eosin stain, × 400 magnification) demonstrates blastic, hyperchromatic and pleomorphic cells of the granulocytic series, consistent with granulocytic sarcoma.
References
1. Meis JM, Butler JJ, Osborne BM, Manning JT. Granulocytic sarcoma in nonleukemic patients. Cancer. 1986; 58:2697–2709.
2. Dusenbery KE, Howells WB, Arthur DC, Alonzo T, Lee JW, Kobrinsky N, et al. Extramedullary leukemia in children with newly diagnosed acute myeloid leukemia: a report from the Children’s Cancer Group. J Pediatr Hematol Oncol. 2003; 25:760–768.
3. Guermazi A, Feger C, Rousselot P, Merad M, Benchaib N, Bourrier P, et al. Granulocytic sarcoma (chloroma): imaging findings in adults and children. AJR Am J Roentgenol. 2002; 178:319–325.
4. Choi EK, Ha HK, Park SH, Lee SJ, Jung SE, Kim KW, et al. Granulocytic sarcoma of bowel: CT findings. Radiology. 2007; 243:752–759.
5. Yilmaz AF, Saydam G, Sahin F, Baran Y. Granulocytic sarcoma: a systematic review. Am J Blood Res. 2013; 3:265–270.
6. Huang XL, Tao J, Li JZ, Chen XL, Chen JN, Shao CK, et al. Gastric myeloid sarcoma without acute myeloblastic leukemia. World J Gastroenterol. 2015; 21:2242–2248.
7. Toma P, Granata C, Rossi A, Garaventa A. Multimodality imaging of Hodgkin disease and non-Hodgkin lymphomas in children. Radiographics. 2007; 27:1335–1354.
8. Patnana M, Sevrukov AB, Elsayes KM, Viswanathan C, Lubner M, Menias CO. Inflammatory pseudotumor: the great mimicker. AJR Am J Roentgenol. 2012; 198:W217–W227.
9. Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 2003; 23:283–304. 456quiz 532.
10. Kim SJ, Kim WS, Cheon JE, Shin SM, Youn BJ, Kim IO, et al. Inflammatory myofibroblastic tumors of the abdomen as mimickers of malignancy: imaging features in nine children. AJR Am J Roentgenol. 2009; 193:1419–1424.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download