Abstract
Lower extremity infection caused by Vibrio vulnificus sepsis is a rapidly progressing fatal condition. Prompt diagnosis followed by early and aggressive treatment with antibiotics and fasciotomy is crucial. In this report, we described lower extremity magnetic resonance (MR) images of three patients with Vibrio vulnificus sepsis. In our cases, MR imaging of lower extremity with Vibrio vulnificus sepsis showed three common findings. First, the MR signal abnormalities appeared simultaneously in all layers, including skin, subcutaneous fat, muscles, and deep fasciae. Second, the inflammation showed symmetry on both legs. Third, none of our cases was accompanied by abscess formation. These imaging features may represent rapid progression of Vibrio vulnificus sepsis and could be helpful for accurate diagnosis, and prompt and aggressive treatment.
Soft-tissue infection by Vibrio vulnificus is divided into two groups, wound infection and sepsis. Wound infections may occur in any area; however, in case of sepsis, which is defined as organisms in the blood, the most common sites of soft tissue manifestations are lower extremities (1). Vibrio vulnificus sepsis commonly occurs in immunocompromised patients who have underlying liver cirrhosis and diabetes mellitus, and is frequently associated with history of eating raw seafood (1). Although soft-tissue infection caused by Vibrio vulnificus occurs rarely, it is one of the most fatal soft-tissue infections. The mortality rate of Vibrio soft-tissue sepsis reportedly ranges from 46% to 79% (1). Therefore, early diagnosis is very essential and urgent surgical debridement with aggressive antibiotic therapy is the most effective treatment.
Magnetic resonance (MR) imaging is the preferred imaging modality in evaluation of soft tissue infection because it provides useful information about extent of inflammation and presence of abscess, thus distinguishing types of musculoskeletal infections (23). It may help in planning treatment strategies for lower extremity infection (3).
Herein, we reported three cases of soft tissue infection in the lower extremity caused by Vibrio vulnificus sepsis with special focus on three common MR imaging findings.
Clinical presentations and disease courses of the three cases were summarized in Table 1. MR imaging findings and histopathologic findings of three cases were summarized in Table 2.
A 61-year-old male presented at the emergency department with pain, tenderness, and claudication of bilateral lower extremities. He demonstrated bilateral patchy erythema and cyanosis from feet to knees (Fig. 1A). Hypotension and high fever was noted. The patient reported eating raw shellfish three days earlier. In addition, he had a history of alcoholic liver cirrhosis.
MR imaging of both lower extremities (Fig. 1B, C) showed abnormal increased signal intensity in all layers of soft tissue including skin, subcutaneous fat, multiple muscles of all compartments, and intermuscular septa of bilateral legs simultaneously on T2-weighted images. Intermuscular septa and adjacent muscles were enhanced on gadolinum enhanced T1-weighted images. There was no abscess formation.
The patient underwent fasciotomy 20 hours after admission. Histologic examination of deep fasciae revealed degeneration of fibrous tissue and multifocal inflammatory cell infiltration (Fig. 1D). The blood culture was positive for Vibrio vulnificus. Despite the fasciotomy and extensive antibiotics, the patient died as a result of progressive septic shock and multi-organ failure.
A 51-year-old male presented with fever, abdominal pain, and watery diarrhea. He also complained of pain, heating sensation, and redness of both legs (Fig. 2A). He reported visiting a beach and eating raw trumpet shells and squids two days earlier. In addition, he had diabetes mellitus and hypertension.
MR imaging of bilateral lower extremities demonstrated multifocal patchy, high signal intensity affecting all layers of soft tissue- multiple muscles, intermuscular septa, overlying subcutaneous fat and skin- on T2-weighted images. It was accompanied by thin enhancement along the intermuscular septa of both legs. Also, there was no abscess formation (Fig. 2B, C).
Fasciotomy was performed 48 hours after admission. Microscopically, deep fasciae revealed acute inflammation (Fig. 2D). Vibrio vulnificus was isolated from the blood culture. After extensive antibiotic therapy, the patient gradually recovered and was discharged a month after admission.
A 49-year-old male with liver cirrhosis was referred to the emergency department for fever and melena. The active gastrointestinal bleeding focus was not found on endoscopy and angiography. After admission, the patient had various sized confluent erythematous patches, bullae, and ecchymoses on his both legs. There was no history of eating raw seafood or seawater exposure.
MR imaging of both lower extremities (Fig. 3A, B) presented high signal intensity lesions in all soft tissue compartment of both legs including skin, subcutaneous fat, multiple muscles, and intermuscular septa with enhancement along intermuscular septa. Abscess was absent in both legs.
Punch biopsy of the skin lesion was performed 12 hours after admission. On microscopic examination, neutrophils were diffusely scattered in deep dermis, subcutaneous fat, and superficial fascia (Fig. 3C). The patient did not have surgery. Vibrio vulnificus was isolated from the blood culture. Despite of antibiotic therapy, sepsis and multi-organ failure progressed and he eventually died.
In the three study patients, MR images were very similar and may be summarized into the three common findings. First, the MR signal abnormalities appeared at all layers of bilateral lower extremities, including skin, subcutaneous fat, muscles, and deep fasciae simultaneously. Second, the lesions showed symmetry on both legs. Third, none of our cases showed abscess formation.
Vibrio vulnificus, first described in 1979, is endemic to warm coastal water with temperature over 20℃ and salt concentrations from 0.7% to 1.6% (4). These organisms are found in warm sea waters and often present in raw seafood, such as raw oysters and shellfish. People with immunocompromized conditions, particularly those with liver cirrhosis, are at high risk for Vibrio vulnificus primary septicemia (5). Cases 1 and 3 with a history of liver cirrhosis, had rapidly worsening sepsis that resulted in death. Vibrio sepsis can manifest as cutaneous lesions characteristically involving the extremities including cellulitis, bullae and ecchymosis (16). These lesions become necrotic and require early surgical debridement or amputation.
Vibrio vulnificus have several virulent and cytotoxic factors that lead to rapid and fatal process (7). Several virulent extracellular toxins and enzymes, such as hemolysin, metalloprotease, collagenase, lipase, and deoxyribonuclease are produced. Hemolysin can form pores in the endothelial cells of blood vessels, causing hemorrhagic bullae. It can induce mast cells to release histamine and activate kinin pathways, resulting in increased vascular permeability and vasodilation. Metalloprotease causes rapid tissue degeneration and necrosis extending through multiple layers with vascular congestion and perivascular neutrophil infiltration (6). These histologic processes were well demonstrated in our cases (Figs. 1D, 2D, and 3C). In addition, the invasive form of Vibrio vulnificus has acid mucopolysaccharide capsule, which confers resistance to immune response of the host (7). These toxic and rapidly destructive characteristics of Vibrio vulnificus might contribute to rapid extension with bilateral and simultaneous multi-layer soft tissue damage, as in our cases. Moreover, none of the three cases had abscess formation in the infected tissue on MR, which might be a result of too rapid progression of inflammation to form the abscess wall.
The bilateral and simultaneous multi-layer soft tissue damage by Vibrio vulnificus infection are distinct from common pyogenic soft tissue infections. Usually, pyogenic soft tissue infection involves exclusively one or some layers of soft tissue, and localized focal area in unilateral extremity in the forms of cellulitis or myositis. Necrotizing fasciitis typically shows non-enhancing portions in or around the abnormally thickened deep fascia (89). Early-stage necrotizing or non-necrotizing fasciitis may show increased signal intensity involving dermis, muscle, and fascia on fluid-sensitive sequences on MRI (8), similar to our three cases of Vibrio infection.
To our knowledge, only one report has described MR imaging findings of soft-tissue infection by Vibrio vulnificus. Lee and Na (10) suggested that characteristic findings of Vibrio infection are necrotizing fasciitis and myositis. They described that the necrotic lesions of muscles and fasciae showed as high signal intensities on T2-weighted images with lack of contrast enhancement, which were obtained after five days of symptom onset, and confirmed by surgery nine days after the symptom onset. However, deep fasciae were enhanced and surrounding non-enhancing area was not observed in all our study cases, possibly because MR images in our cases were performed earlier than reported by Lee and Na. (10)
In case 3, despite lack of evidence of raw seafood consumption or seawater contact, the skin lesion and septic condition, along with MR imaging abnormality affecting multi-layer soft tissue symmetrically in both legs, should have led to an earlier presumptive diagnosis of Vibrio sepsis with need for urgent fasciotomy.
In conclusion, lower extremity MR images of Vibrio vulnificus sepsis in our three cases have common findings showing bilateral symmetric, multi-layer involvement without abscess formation. These findings could be helpful in the prompt diagnosis of Vibrio vulnificus sepsis especially in immunocompromised patients with recent history of seawater or seafood exposure.
Figures and Tables
Fig. 1
A 61-year-old man diagnosed as Vibrio vulnificus sepsis (case 1).
A. Photograph of bilateral lower extremities demonstrates multifocal erythematous patches, ecchymoses, and cyanotic skin lesions. The upper boundary of the lesion is marked, to determine superior spread of the lesion.
B, C. Axial T2-weighted image (B) shows abnormal increased signal intensity in skin, subcutaneous fat, multiple muscles of all compartments, and transverse intermuscular septa (arrows) of both legs. Gadolinum enhanced fat-suppressed T1-weighted image (C) shows enhancement of superficial fascia and transverse intermuscular septa (arrows). There is no abscess in both lower extremities.
D. Histologic image of fascial layer of the leg reveals degeneration of fibrous tissue (open arrow) and multifocal neutrophil infiltrations (arrow) (hematoxylin and eosin stain, × 400).
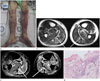
Fig. 2
A 51-year-old man diagnosed as Vibrio vulnificus sepsis (case 2).
A. Photograph of bilateral lower extremities shows multiple petechiae and patches.
B, C. Axial T2-weighted image (B) shows multifocal patchy high signal intensity in multiple muscles, intermuscular septa (arrows), and overlying subcutaneous fat and skin of both legs. Gadolinum enhanced fat-suppressed T1-weighted image (C) demonstrates thin enhancement along the transverse and posterior intermuscular septa (arrows). There is no abscess in both lower extremities.
D. Histologic image of fascia of the leg reveals vascular congestion in the fascia with perivascular neutrophil infiltrations (open arrows) (hematoxylin and eosin stain, × 100).
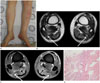
Fig. 3
A 49-year-old man diagnosed as Vibrio vulnificus sepsis (case 3).
A, B. Axial T2-weighted MR image (A) shows high signal intensity affecting skin, subcutaneous fat, and multiple muscles, and intermuscular septa (arrows) of both legs. Gadolinum enhanced fat-suppressed T1-weighted image (B) shows mild enhancement of bilateral transverse intermuscular septa (arrows). There is no abscess in both lower extremities.
C. Histologic specimen of punch biopsy of the skin lesion in the leg reveals diffusely scattered neutrophils in subcutaneous fat layer (arrow), superficial fascia (dashed arrow), and vascular wall of deep dermis (open arrow) (hematoxylin and eosin stain, × 40). Square box at right below shows histologic specimen (× 12 magnification).
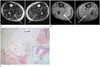
Table 1
Summary of Clinical Presentations and Disease Course of Three Cases

Table 2
Summary of MR Findings and Histopathologic Findings of Three Cases

References
1. Park SD, Shon HS, Joh NJ. Vibrio vulnificus septicemia in Korea: clinical and epidemiologic findings in seventy patients. J Am Acad Dermatol. 1991; 24:397–403.
2. Turecki MB, Taljanovic MS, Stubbs AY, Graham AR, Holden DA, Hunter TB, et al. Imaging of musculoskeletal soft tissue infections. Skeletal Radiol. 2010; 39:957–971.
3. Paz Maya S, Dualde Beltrán D, Lemercier P, Leiva-Salinas C. Necrotizing fasciitis: an urgent diagnosis. Skeletal Radiol. 2014; 43:577–589.
4. Chiang SR, Chuang YC. Vibrio vulnificus infection: clinical manifestations, pathogenesis, and antimicrobial therapy. J Microbiol Immunol Infect. 2003; 36:81–88.
5. Haq SM, Dayal HH. Chronic liver disease and consumption of raw oysters: a potentially lethal combination--a review of Vibrio vulnificus septicemia. Am J Gastroenterol. 2005; 100:1195–1199.
6. Strom MS, Paranjpye RN. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2000; 2:177–188.
7. Horseman MA, Surani S. A comprehensive review of Vibrio vulnificus: an important cause of severe sepsis and skin and soft-tissue infection. Int J Infect Dis. 2011; 15:e157–e166.
8. Chaudhry AA, Baker KS, Gould ES, Gupta R. Necrotizing fasciitis and its mimics: what radiologists need to know. AJR Am J Roentgenol. 2015; 204:128–139.
9. Kim KT, Kim YJ, Won Lee J, Kim YJ, Park SW, Lim MK, et al. Can necrotizing infectious fasciitis be differentiated from nonnecrotizing infectious fasciitis with MR imaging? Radiology. 2011; 259:816–824.
10. Lee JH, Na JB. MR findings of infectious myositis caused by vibrio vulnificus: case report. J Korean Radiol Soc. 2003; 48:285–288.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download