Abstract
Pleural malignant mesotheliomas arise from mesothelial cells in the pleura. They are characterized as diffuse or localized malignant mesotheliomas (LMM). Diffuse malignant mesotheliomas spread diffusely along pleural surfaces, while LMM are well-circumscribed nodular lesions with no gross or microscopic diffuse pleural spreading. Therefore, LMM can be radiologically confused with solitary fibrous tumors of the pleura (SFTP), which commonly presents as a solitary, well-demarcated peripheral mass abutting the pleural surface upon the completion of a computed tomography (CT). Therefore, this study reports on a 63-year-old female patient with a pathologically-proven LMM of the pleura, mimicking a benign SFTP upon having a chest CT. Although LMM is extremely rare, FDG PET/CT should be recommended for adequate tumor management in order to avoid misdiagnosing the tumor as a benign SFTP when an interfissural or pleural-based mass is seen on the chest CT.
Malignant mesotheliomas are malignant tumors that arise from mesothelial cells in the pleura, pericardium, and peritoneum. These tumors are further characterized as diffuse malignant mesotheliomas (DMM) or localized malignant mesotheliomas (LMM). LMM share the same microscopic, histochemical, immunohistochemical and ultrastructural features that DMM portray (1). However, DMM spreads diffusely along the pleural surfaces, while LMM are well circumscribed nodular lesions with no gross or microscopic diffuse pleural spreading. Due to these characteristics, LMM can be radiologically confused with other benign pleural tumors. For instance, on a computed tomography (CT), an LMM appears similar to solitary fibrous tumors of the pleura (SFTP), which commonly presents as a solitary, well-demarcated peripheral mass abutting the pleural surface. Therefore, this study presents a case of a CT scan that shows an LMM mimicking a benign SFTP.
A 63-year-old female patient was referred for an enlarging, mass-like opacity in the right upper lung zone, which was detected on a routine chest radiograph. She presented with dyspnea on exertion, but did not complain of any other respiratory symptoms including fever, cough, or sputum production. She did however have a seven-year history of hypertension. There was no history of smoking or occupational exposure to asbestos. A complete blood count, cardiac markers, D-dimer and serum tumor markers were all within normal limits. An electrocardiogram was normal, while an echocardiogram revealed a grade 2 mitral regurgitation and grade 1 diastolic dysfunction.
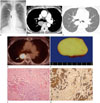
During an admission for a colon polypectomy two years prior, a routine chest radiograph revealed a 3.6 cm ovoid mass in the right paraspinal area inferior to the posterior junctional line, and no further evaluation at that time. On a follow-up chest radiograph, the mass had slightly increased in size to 4.0 cm, which was suggestive of a benign posterior mediastinal tumor (Fig. 1A). A subsequent contrast enhanced chest CT scan revealed a well-defined, ovoid, 2.5 × 3.2 × 3.8 cm interfissural mass in a mediastinal reflection at the same level of the tracheal carina (Fig. 1B, C). The mass was homogeneously mildly enhanced after a contrast injection. This raised the possibility of a benign SFTP rather than a posterior mediastinal tumor such as a neurogenic tumor. However, upon completion of a positron emission tomography with a 2-deoxy-2-fluorine-18fluoro-D-glucose integrated with a CT (FDG PET/CT), there was positive FDG uptake of the mass with 8.5 of maximum standardized uptake value (SUVmax) (Fig. 1D). This finding is highly suggestive of malignancy. There was neither an abnormal mediastinal nor pulmonary FDG uptake. For simultaneous confirmative diagnosis and treatment, the patient underwent video-assisted thoracoscopic surgery. Intraoperatively, the mass, which originated from the parietal pleura with a membranous stalk of 0.5 cm in length, was located in the right major interlobar fissure, and was completely resected without complication. Histopathologic examination of the interfissural mass revealed a LMN of the biphasic type, consisting of epithelioid and sarcomatoid components (Fig. 1E, F). Immunohistochemistry revealed positive cytokeratin and calretinin staining, and a negative thyroid transcription factor-1 and factor 13a staining (Fig. 1G). The patient recovered well, and has not had any local recurrences or distant metastases for two years since the surgery.
Pleural LMM is extremely rare. Approximately 60 cases of pleural LMM have been reported in English literature. Given the small number of reported cases, the characteristics of pleural LMM are not well known. On a CT scan, pleural LMM mainly presents as a solitary and well-circumscribed tumor (23); rib or chest wall invasion may be shown. There is typically small or no pleural effusion. In most previous cases, contrast enhanced CT findings included heterogeneous or irregular enhancement otherwise our case, which corresponded to internal necrosis or hemorrhage (3). With regard to the gross pathology of LMM, a sessile form is a more common but pedunculated mass in our case. In addition, LMM more commonly originated from the parietal pleural than from the visceral pleura. The tumor size varied, ranging 0.7 cm to 19 cm (4). In the present case, after a CT scan was conducted a tumor presenting as a solitary, well-circumscribed mass with homogenous enhancement was found. It is believed that this discordance with the literature may be due to the fact that our case included a smaller tumor than that which was previously reported. Larger tumors tend to be more heterogeneous than smaller ones.
There were several follow-up studies of LMM. Takahashi et al. (5) reported a case of pleural thickening that developed into a 4.5 cm mass over 14 months. Makimoto et al. (6) reported a case of a small nodule that grew into a massive tumor 5 months later. Zardawi et al. (7) reported a case involving a mass that increased twice in size over 3 months, from 4 cm to 8 cm. In the present case, however, the mass only grew by 10% over 24 months. The growth rate of pleural LMM appears to be an independent factor to determine malignancy.
It is important to differentiate an LMM from a benign SFTP because an SFTP is the most common solitary pleural neoplasm. In the past, a benign SFTP was considered a benign tumor originating from mesothelial cells. It was referred to as ‘benign mesothelioma’ or ‘localized fibrous mesothelioma’. More recently, however, a benign SFTP is thought to originate from a ubiquitous interstitial stem cell (mesenchymal cell), rather than from a mesothelial cell. Histologically, SFTP is characterized as a spindle cell neoplasm that mimics sarcomatoid mesothelioma. Immunohistochemical studies such as cytokeratin or CD34 are helpful for the differentiation of SFTP and LMM. SFTP is positive to CD34 and negative to cytokeratin, while LMM is negative to CD34 and positive to cytokeratin (4). In addition, the differentiation of benign and malignant SFTP is also important and based on mitotic figures, nuclear pleomorphism, necrosis or cellularity. This is due to the fact that both benign and malignant SFTPs are typically well-defined, attenuated soft-tissue masses on CTs. Depending on the tumor size small SFTPs are usually homogeneous. In contrast, large SFTPs are typically heterogeneous and intensely enhanced. Imaging features that favor malignancy include size > 10 cm, extensive necrosis or hemorrhage, pleural effusion, heterogeneity and mass effect (8). However, these CT findings of SFTP can be also found in LMM cases. Therefore, it may be difficult to distinguish SFTP and LMM based on these characteristics.
In several studies regarding the usefulness of FDG-PET to differentiate benign from malignant SFTP, benign SFTP usually revealed negative or low levels of FDG uptake (SUVmax < 2.5), while malignant SFTP revealed a relatively high FDG uptake. Most prior FDG-PET studies of LMM found intense FDG uptake, of which SUVmax were reported as 6.6–15.1 (910). Therefore, FDG-PET may be helpful to differentiate benign SFTP from either malignant SFTP or LMM. In the present case, despite the typical benign CT findings, including no local invasion, and no metastasis and slow growth, there was a concern for malignancy because the SUVmax was 8.5. Researchers of this study propose that FDG-PET could be helpful for making a diagnosis of malignancy, regardless of the benign nature of the pleural mass upon completion of the CT.
In conclusion, this study reported a case of pleural LMM that mimicked a benign pleural tumor upon conducting a CT scan and demonstrated positive FDG uptake. Although LMM is extremely rare, a FDG PET/CT should be recommended for adequate tumor management in order to avoid misdiagnosing the tumor as a benign SFTP when an interfissural or pleural-based mass is seen on a chest CT.
Figures and Tables
Fig. 1
A 63-year-old woman with localized malignant mesothelioma.
A. The chest radiograph shows a 4 cm ovoid mass in the right paraspinal area inferior to the posterior junctional line (arrows).
B, C. Mediastinal (B) and lung (C) window images of contrast enhanced chest CT scan reveal a well-defined ovoid 3.8 cm pleural based mass (arrows) in a mediastinal reflection in the posterior aspect of the tracheal carina. The mass shows a mild homogeneous enhancement (arrow in B) and an interfissural location (arrow in C).
D. FDG PET/CT scan shows a positive FDG uptake of the mass with 8.5 of the maximum standardized uptake value (SUVmax) (arrow).
E. Photograph of the surgical specimen grossly reveals a well demarcated solid grayish mass.
F. Photomicrograph of the surgical specimen shows atypical mesothelial cell proliferation with papillary and solid pattern. Cell groupings and cytologic atypism are also present, which is suggestive of malignancy (hematoxylin & eosin, × 100).
G. Photomicrographs of the surgical specimen show a strong positive reaction for calretinin, consistent with mesothelial origin (immunohistochemical stain, × 200).
References
1. Allen TC, Cagle PT, Churg AM, Colby TV, Gibbs AR, Hammar SP, et al. Localized malignant mesothelioma. Am J Surg Pathol. 2005; 29:866–873.
2. Tanzi S, Tiseo M, Internullo E, Cacciani G, Capra R, Carbognani P, et al. Localized malignant pleural mesothelioma: report of two cases. J Thorac Oncol. 2009; 4:1038–1040.
3. Yao W, Yang H, Huang G, Yan Y, Wang H, Sun D. Massive localized malignant pleural mesothelioma (LMPM): manifestations on computed tomography in 6 cases. Int J Clin Exp Med. 2015; 8:18367–18374.
4. Okamura H, Kamei T, Mitsuno A, Hongo H, Sakuma N, Ishihara T. Localized malignant mesothelioma of the pleura. Pathol Int. 2001; 51:654–660.
5. Takahashi H, Harada M, Maehara S, Kato H. Localized malignant mesothelioma of the pleura. Ann Thorac Cardiovasc Surg. 2007; 13:262–266.
6. Makimoto G, Fujiwara K, Fujimoto N, Yamadori I, Sato T, Kishimoto T. Phrenic nerve paralysis as the initial presentation in pleural sarcomatoid mesothelioma. Case Rep Oncol. 2014; 7:389–392.
7. Zardawi SJ, Li BT, Zauderer MG, Wang JW, Atmore BB, Barnes TA, et al. Localized malignant pleural mesothelioma with renal metastasis. Oxf Med Case Reports. 2015; 2015:170–172.
8. Abu Arab W. Solitary fibrous tumours of the pleura. Eur J Cardiothorac Surg. 2012; 41:587–597.
9. Ko HM, Kamil ZS, Geddie WR. Microcystic variant malignant mesothelioma presenting as a localized paraspinal mass. Cytojournal. 2014; 11:16.
10. Morimoto D, Fujimoto N, Nishi H, Asano M, Fuchimoto Y, Ono K, et al. Malignant pleural mesothelioma localized in the thoracic wall. J Thorac Oncol. 2012; 7:e21–e22.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download