Abstract
Developmental venous anomaly (DVA) is a common congenital venous malformation characterized by dilated medullary veins in caput medusa configuration and a draining vein. Despite the high incidence of DVAs, they are benign anatomic variations and rarely cause symptoms. Here, we report computed tomography and magnetic resonance imaging findings with perfusion images of acute infarction from underlying DVA in a 63-year-old female patient who presented with acute onset of neurologic symptoms and recovered without any neurologic deficit.
Developmental venous anomaly (DVA) is a common congenital malformation characterized by dilated medullary veins (12) configured in the caput medusa and a draining vein. The incidence of DVAs is bigh, but they are benign anatomic variations and rarely display symptoms. Here, we report findings by computed tomography (CT) and magnetic resonance imaging (MRI) with perfusion images of acute infarction from underlying DVA in a 63-year-old female patient who presented with acute onset of neurologic symptoms and recovered without any deficit remaining.
DVAs are quite common with a reported incidence between 0.7 and 2.56% in the general population (3) but unlike other congenital malformations, they are rarely symptomatic and in most cases diagnosed by chance. The reasons why they rarely cause symptoms can be found ubeither mechanical compression to adjacent intracranial structures or misbalance of the blood flow (4).
A 63-year-old woman showing up in an emergency room complained about an abrupt onset of right side motor weakness and sensory changes. These symptoms were followed by dysarthria the day before her visit. However, all the symptoms disappeared spontaneously by the time she arrived at the emergency room and at the time of the initial physical examination, which included motor and sensory grades andwere within normal limits.
She was in good health prior to the event and had no past medical history. Neither was there anything noteworthy about her family history. Further evaluation included a conventional echocardiogram. The following additional laboratory studies were either negative or within normal range: antinuclear and anticardiolin antibodies, antithrombin III, factor V Leiden mutation, protein C, protein S, activated protein C resistance, and homocysteine.
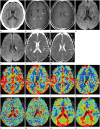
Initial ordinary CT images of the brain displayed a subtle hypodense area in the left basal ganglia, but no mass effect or acute hemorrhagic focus was identified (Fig. 1A).
On the following day, MRI was conducted and gadolinium-enhanced T1-weighted images showed growing medullary veins at the left putamen in a characteristic ‘caput medusa’ configuration with a collector vein draining to the surface of the left lateral ventricle, which confirmed the DVA diagnosis (Fig. 1B, C). This location corresponded to the hypodense area noted on the CT image. Diffusion weighted images (DWI) revealed the presence of an area of bright signal intensity (SI) in the left basal ganglia and periventricular white matter nearby the draining vein, which demonstrated decreased SI on an diffusion coefficient (ADC) map (Fig. 1D-G). There was no discernible hemorrhagic transformation on susceptibility weighted images. Magnetic resonance angiography demonstrated no significant stenosis or intracranial artery occlusion. There was no increased focal mean flow velocity as shown by a transcranial doppler.
The perfusion study showed relatively increased cerebral blood volumes (rCBV) and cerebral blood flows (rCBF) with an associated prolonged mean transit time (MTT) and time to peak (TTP), as compared to contralateral normal parenchyma, representing venous congestion (Fig. 1H-O).
The patient's symptoms resolved spontaneously within 24 hours after onset without neurologic deficits. The patient continued to be symptom-free for at least 11 months following initial presentation.
Currently, DVAs are regarded as a primary dysplasia of capillaries and small transcerebral veins or a compensatory mechanism caused by an arrest of normal venous development during-embryogenesis (7). They are thought to represent variations of parenchymatas' venous drainage and surgical resection should be avoided, because when they are drained no other normal venous discharging system exists (12).
Despite the DVAs' high incidence, they are benign anatomic variations and rarely cause symptoms due to either mechanical compression to adjacent intracranial structures, which may result in hydrocephalus, tinnitus, hemifacial spasms, and trigeminal neuralgia or a misbalance of the blood flow (4). Flow related complications can be characterized as a misbalance of the blood's in- and outflow in the DVA system, which raises the pressure in the DVAs, either due to an increase of the former or a restriction of the latter (4). They may also give rise to acute symptoms which can mimic arterial stroke due to the draining vein's restricted outflow and venous congestion, which might cause an increase of complications such as hemorrhages or infarctions. Ruíz et al. (8) reported complications in 19 cases with symptomatic DVAs (venous ischemic infarction. 53%, parenchymal hemorrhage, 37%, and subarachnoid and intraventricular hemorrhages, 5%). DVAs also frequently coexist with other types of vascular malformations, such as cavernous ones and they may be associated with a higher hemorrhagic risk. There are several reported cases of DVAs with thrombotic obstruction of draining veins, resulting in venous infarction with or without parenchymal hemorrhage (568). Thrombosis of an DVA's draining vein leading to venous brain infarction is a rare complication and an infarction remained nonhemorrhagic, if early recanalization is ach-ieved (9).
According to Pereira et al. (4), mechanical and increased inflow types of flow-related symptomatic DVA can be excluded in our case, since there was no intracranial structure causing mechanical compression or cerebral arteriovenous malformation which may also result in an increased inflow of DVA. In our case, there was no discernible venous thrombosis as found in the imaging studies and we assumed there a possibility of venous infarction with early recanalization of thrombosed vein might exist; this implies a decreased outflow type of flow-related symptomatic DVA, considering that there is no hemorrhage associated with infarction and that the acute onset of the patient's symptoms happened without a significant past medical history.
DVAs are thought to lack smooth muscle cells and elastic connective tissue resulting in less flexibility to changes of hemodynamic disturbances (56). Truwit (1) reported that a focal stenosis of the draining vein is possible at the point where it penetrates the dura which can cause lessened compliance, increased resistance to flow, and reduced capacity of the vessel for pressure change adjustments. In addition, a relative volume overload, previous hemorrhages, and chronic cerebral ischemia or venous hypertension can result when a large parenchymal territory is drained by a DVA. As a result, it has been suggested that imaging abnormalities of high SI on T2 FLAIR images, higher ADC values, and perfusion alterations in the DVAs' vicinity can be explained by chronic cerebral ischemia, resulting from venous congestion (10). The pattern of perfusion abnormalities in our case was similar to previously reported results, e.g., an increased rCBV and rCBF with prolonged MTT and TTP, implicating venous congestion with delayed perfusion (10).
Regarding DWI and ADC maps, focal SI change of diffusion restriction may be indistinguishable from arterial infarction, but underlying DVA can be identified by enhanced T1-weighted images. In addition, DVA-associated perfusion abnormalities may represent its role regarding venous congestion.
Treatment principles of acute infarctions related to DVA have not been established, but a number of case series has reported favorable outcomes after anticoagulation therapy, particularly in instances of the draing vein associated with thrombus (567). Due to differences in the pathophysiology of venous and arterial stroke, venous infarction may not have the same prognostic value as the arterial infarct and it may be completely reversible (6).
In conclusion, even though DVAs commonly encounter insignificant anatomic variants, they rarely cause venous infarction with acute neurologic symptoms; characteristics of imaging features by perfusion studies may provide a clue to diagnosis. The understanding of and familiarity with imaging findings of complicated DVAs is essential for accurate diagnoses with different kinds of management of and prognoses about arterial infarction.
Figures and Tables
Fig. 1
Venous infarction of developmental venous anomaly in a 63-year-old woman who presented with an acute onset of right side motor weakness and sensory changes.
Axial, non-enhanced (conventional) computed tomography (A) revealed a subtle hypodense area (black arrow) in the left basal ganglia compared to the contralateral side. Gadolinium-enhanced T1-weighted axial images displayed the enhancement of medullary veins (white arrow, B) at the left putamen in the ‘caput medusa’ configuration with a collector vein (white arrowhead, C) draining to the eft lateral ventricle's surface. Diffusion weighted images (D, E) showed hyperintense lesions in left basal ganglia and periventricular white matter nearby the draining vein. A corresponding diffusion coefficient map (F, G) demonstrated diffusion restrictions, indicating acute infarction.
Perfusion images revealed a regional increase of relative cerebral blood flow (H, I) and of cerebral blood volume (J, K), surrounding the developmental venous anomaly, with a prolongation of mean transit time (L, M) and time to peak (N, O).
References
1. Truwit CL. Venous angioma of the brain: history, significance, and imaging findings. AJR Am J Roentgenol. 1992; 159:1299–1307.
2. Lasjaunias P, Burrows P, Planet C. Developmental venous anomalies (DVA): the so-called venous angioma. Neurosurg Rev. 1986; 9:233–242.
3. Töpper R, Jürgens E, Reul J, Thron A. Clinical significance of intracranial developmental venous anomalies. J Neurol Neurosurg Psychiatry. 1999; 67:234–238.
4. Pereira VM, Geibprasert S, Krings T, Aurboonyawat T, Ozanne A, Toulgoat F, et al. Pathomechanisms of symptomatic developmental venous anomalies. Stroke. 2008; 39:3201–3215.
5. Griffiths D, Newey A, Faulder K, Steinfort B, Krause M. Thrombosis of a developmental venous anomaly causing venous infarction and pontine hemorrhage. J Stroke Cerebrovasc Dis. 2013; 22:e653–e655.
6. Masson C, Godefroy O, Leclerc X, Colombani JM, Leys D. Cerebral venous infarction following thrombosis of the draining vein of a venous angioma (developmental abnormality). Cerebrovasc Dis. 2000; 10:235–238.
7. Field LR, Russell EJ. Spontaneous hemorrhage from a cerebral venous malformation related to thrombosis of the central draining vein: demonstration with angiography and serial MR. AJNR Am J Neuroradiol. 1995; 16:1885–1888.
8. Ruíz DS, Yilmaz H, Gailloud P. Cerebral developmental venous anomalies: current concepts. Ann Neurol. 2009; 66:271–283.
9. Konan AV, Raymond J, Bourgouin P, Lesage J, Milot G, Roy D. Cerebellar infarct caused by spontaneous thrombosis of a developmental venous anomaly of the posterior fossa. AJNR Am J Neuroradiol. 1999; 20:256–258.
10. Jung HN, Kim ST, Cha J, Kim HJ, Byun HS, Jeon P, et al. Diffusion and perfusion MRI findings of the signal-intensity abnormalities of brain associated with developmental venous anomaly. AJNR Am J Neuroradiol. 2014; 35:1539–1542.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download