Abstract
A 24-year-old man presented with dyspnea on exertion and intermittent blood-tinged sputum. He was diagnosed with aberrant systemic arterial supply to the normal lung (ASANL) based on the results of imaging studies. The patient was successfully treated with transarterial embolization using coils and a vascular plug and his symptoms disappeared during the follow-up. Herein, we reported the imaging findings of ASANL, differential diagnoses, and its treatment options. In addition, we reviewed the relevant literature.
Aberrant systemic arterial supply to the normal lung (ASANL) is a rare disease. Although most patients with ASANL are asymptomatic, the presenting symptoms are cardiac murmur in childhood, and recurrent hemoptysis, dyspnea on exertion, and congestive heart failure in adults (1). Diagnosis is relatively easy with chest computed tomography (CT) (12). However, treatment options vary, including surgical resection of the involved lung and surgical ligation of the aberrant artery, and endovascular treatment has been recently introduced (3). We reported a case of a 24-year-old man with ASANL who was treated by the endovascular method. In addition, we reviewed the relevant literature.
A 24-year-old man presented with dyspnea on exertion and intermittent blood-tinged sputum. Dyspnea had started in his childhood, and was aggravated with intermittent blood-tinged sputum for the past 2–3 years. He was a social smoker (1 cigarette a day), and had no specific history.
Chest CT showed large tortuous vessels arising from the descending thoracic aorta in the basal segments of the left lower lobe (Fig. 1A). The largest diameter of the vessel at the origin was 1.4 cm. There were no findings to suggest hypertrophied systemic artery associated with chronic inflammation. In contrast to scimitar syndrome, the lungs were not hypogenetic, and the left lower lobar pulmonary vein was dilated and normally drained to the left atrium (Fig. 1B). Courses of the bronchial tree were normal, and thickening of the interstitial markings with ground-glass opacities were observed. These findings were compatible with ASANL.
For evaluation of cardiac and pulmonary complications, echocardiography, lung perfusion scan, and pulmonary function test were conducted. Echocardiography indicated mild left ventricular enlargement with normal ejection fraction. Lung perfusion scan showed severe hypoperfusion in the left lower lobe. Mild obstructive lung disease was suspected on pulmonary function test.
Treatment options include surgical treatment such as wedge resection of the involved lung, and surgical ligation of the aberrant systemic artery (ASA), and endovascular treatment. Endovascular treatment was chosen considering his young age and potential for early recovery, and informed consent was obtained.
The right femoral artery and the left femoral vein were punctured under local anesthesia. Left pulmonary angiography sh-owed a hypoplastic left lower pulmonary artery with decreased perfusion in the left lower lung zone (LLLZ). On thoracic aortography, an ASA supplying the LLLZ was observed. A 5 Fr Yashiro catheter (Radifocus®; Terumo Corp., Tokyo, Japan) was advanced to the origin of the ASA, and selective aberrant arteriography showed large arterial bran-ches supplying the LLLZ. We also observed an enlarged left lower pulmonary vein, which drained to the left atrium. However, there was no direct shunt vessel. Transarterial embolization (TAE) was performed from the distal branches of the ASA, using twelve 6-mm, and eleven 8-mm platinum coils (Nester®; Cook, Bloomington, IN, USA) and one 10-mm fibered IDC coil (Interlock®; Boston Scientific, Natick, MA, USA). After coil embolization, the proximal portion of the ASA was embolized using a 14-mm Amplatzer Vascular Plug® type II (AGA Medical Corporation, Golden Valley, MN, USA), to cover the largest portion of the ASA at its origin. Post-embolization thoracic aortography indicated absence of blood flow from the ASA to the left lower pulmonary vein. However, new development of hypertrophied left bronchial artery with an arteriovenous shunt was noted in the LLLZ. This artery was targeted with a 1.98 Fr microcatheter and embolized using two 3-mm microcoils (Nester) and 1000–1400-µm gelfoam particles (Cali-Gel®; Alicon Pharm SCI&TEC Co., Ltd., Hangzhou, China). After embolization, the hypertrophied left bronchial artery was not observed (Fig. 1C).
The patient complained of chest pain, fever, and chills for 6 days, which improved with supportive care. One week after TAE, his symptoms disappeared and he was discharged.
The patient was asymptomatic during the 1-year follow-up. Follow-up chest CT at 4-months post-TAE showed decreased dilation of the distal branches of the ASA, and decreased size of the left lower lobar pulmonary vein (Fig. 1D).
ASANL is a rare congenital anomaly (1). Diagnosis of ASANL is relatively easy by imaging studies, especially CT (12). Vascular structures from the descending thoracic aorta in the left lower lobe with normal bronchial distribution are common findings of ASANL (2). On conventional angiography, a dilated ASA supplies the LLLZ and drains into the left atrium through the inferior pulmonary vein in patients with ASANL (34). The pulmonary arterial supply in the affected segments may be normal or absent (5).
In systemic arterial supply to the lungs, especially in adults, hypertrophied normal systemic arteries associated with chronic inflammation and aberrant systemic arteries in congenital anomalies are considered as differential diagnoses (6), for e.g., pseudosequestration shows normal bronchial tree and pulmonary artery with other evidences of chronic inflammation. Congenital anomalies with ASA include scimitar syndrome, bronchopulmonary sequestration, and ASANL. Anomalous venous return with hypogenetic lung are suggestive of scimitar syndrome; whereas, bronchopulmonary sequestration and ASANL are differentiated based on the occurrence of abnormal or normal br-onchial tree, respectively. Fig. 2 shows a simplified algorithm for the differential diagnosis.
Treatment is recommended in symptomatic and asymptomatic patients with ASANL because of the risk of congestive heart failure, recurrent severe infections, and hemorrhagic complications (12). Conventionally, surgical treatment has been the standard therapeutic option (2). In pulmonary sequestration, resection of a sequestered lung is favored because of the risk of recu-rrent infection; whereas, surgical ligation of the ASA alone may be sufficient in cases of ASANL. Because TAE is a well-established treatment in patients with hemoptysis, and surgical ligation of the ASA is indicated in ASANL, embolization can be adjusted in ASANL (35). Since Brühlmann et al. (3) first described a 51-year-old man with ASANL who was successfully treated by coil embolization, 19 cases treated with TAE have been reported in the English literature (Table 1). Brühlmann et al. (3) report a low risk of post-embolization pulmonary infarction because of dual arterial supply to the affected lung parenchyma. Abe et al. (5) reported cases of the complete type of ASANL that lack normal pulmonary arterial supply to the involved area, of which, only one case showed asymptomatic transient pulmonary infarction. Saida et al. (7) suggest that the embolized area has low risk of infarction because tissue permeability or microcirculation through the persistent bronchial arteries preserves the perfusion of tissues. Nevertheless, proximal occlusion with coils or vascular plugs is advocated. Distal embolization using particles, such as gelatin sponge particles or polyvinyl alcohol, should be avoided to minimize the risk of ischemic injury (5). In our case, the ASA was embolized with large sized coils and proximal occlusion was performed using a vascular plug.
A rare case of ASANL with two feeders including a normal systemic artery and ASA supplying the affected lung zone was reported (4). Unlike previously reported ASANL cases, we detected a newly developed hypertrophied bronchial artery soon after the ASA-embolization. Similar phenomena have been reported in the literature on embolization of pulmonary arteriovenous malformations (PAVMs) (89). In our case, the hypertrophied bronchial artery was possibly a preexisting lesion exaggerated by relative ischemia, or a new lesion caused by procedure-related local ischemia. It is likely that the bronchial artery to the involved area underwent compensatory hypertrophy to preserve tissue perfusion. As reported in PAVMs, a hypertrophied bronchial artery can cause recurrent hemoptysis, and therefore, requires treatment. In our patient, the proximal portion of the suddenly developed bronchial artery was embolized with coils and large-diameter gelfoam particles to preserve tissue perfusion through collaterals and minimize the risk of ischemic injury.
Post-embolization syndrome is the most common complication of TAE of ASANL, and can be controlled conservatively. Other reported complications included recurrent bleeding due to developed collaterals or residual arterial supply, and some respiratory symptoms, which were successfully treated. Along with pulmonary infarction, secondary infection and fistula are theoretically possible, but have not been reported to date (10).
Long-term results after TAE for ASANL remain unclear. Previous reports indicate that patients show improvement during the follow-up periods ranging from 6 months to 6 years. Additional long-term studies are clearly required.
Our patient was successfully treated with TAE. Brillet et al. (9) reported development of a systemic collateral supply after embolotherapy of PAVM. They showed that enlargement of a systemic artery was observed more frequently in patients with clinical and/or radiological features suggesting post-treatment lung infarction, than in those without these features. Although our patient has been asymptomatic for 1 year, close and long-term follow-up is required.
Figures and Tables
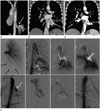 | Fig. 1Aberrant systemic arterial supply to the normal lung treated with transarterial embolization in a 24-year-old man.
A, B. Initial chest CT. Volume rendering image (A) shows a 14-mm-sized, large tortuous vessel (arrow) arising from the descending thoracic aorta and supplying the basal segments of the left lower lobe. On lung window setting image, thickening of the interstitial markings with ground glass opacities, consistent with pulmonary congestion, was noted in the left lower lobe; courses of the bronchial tree in both lungs were normal (not shown). On coronal reformatted image (B), the left lower lobar pulmonary vein is dilated, as compared to the right lower lobar pulmonary vein (curved arrow), and normally drains to the left atrium.
C. Images of conventional angiography (upper panel) and transarterial embolization (lower panel). Left pulmonary angiography shows hypoplasia or absence of the left lower pulmonary artery and decreased perfusion in the left lower lung zone (black arrows). Initial thoracic aortography and selective angiography of the aberrant systemic artery reveal an aberrant systemic artery (white arrows) arising from the descending thoracic aorta and supplying the left lower lung zone. On delayed phase, the enlarged left lower pulmonary vein (open arrow) that drains to the left atrium, is observed. On post-embolization aortography, the aberrant systemic artery is no longer visualized, and hypertrophied left bronchial artery (curved arrow) with arteriovenous shunt in the left lower lung zone, which was not revealed on the initial thoracic aortography, is observed. The left bronchial artery was selected, and embolization was conducted using microcoils and gelfoam particles. On final post-embolization aortography, the aberrant systemic artery and the hypertrophied bronchial artery are obliterated.
D. Follow-up chest CT image at 4 months after treatment. On the coronal reformatted image after treatment, the left lower lobar pulmonary vein is decreased in diameter.
|
 | Fig. 2A simplified algorithm for differential diagnosis of systemic arterial supply to the lung. The presence or absence of ancillary findings of chronic inflammation, abnormal venous return, and courses of the bronchial tree should be checked for differential diagnosis. |
Table 1
Reported Cases of Aberrant Systemic Arterial Supply to the Normal Lung which were Treated with TAE in the English Literature

| Authors | Year of Publication | Sex/Age | Clinical Manifestations | Origins of Systemic Vessels | Pulmonary Artery in Involved Lung Site | Site of Drainage of Lung | Management | Follow-Up Period | Complication after TAE |
|---|---|---|---|---|---|---|---|---|---|
| Jariwala et al. (4) | 2014 | F/7 mo | Failure to thrive | IMA, descending thoracic aorta | Normal | Entire left lung | 2-staged TAE with coils and vascular plugs | 6 months | None |
| Kim et al. (1) | 2014 | M/22 | Recurrent hemoptysis | Descending thoracic aorta | Not mentioned | Right lower lobe | TAE with vascular plugs | 28 months | Post-embolization syndrome |
| Sugihara et al. | 2013 | F/67 | Incidental finding on CT | Descending thoracic aorta | Absent | Left lower lobe | TAE with coils under balloon occlusion | 12 months | None |
| Anil et al. (10) | 2012 | M/23 | Recurrent hemoptysis | Descending thoracic aorta | Absent | Left lower lobe | TAE with coils and cyanoacrylate glue | 6 months | Chest discomfort for 2 days |
| Canyigit et al. | 2011 | M/53 | Acute chest pain | Descending thoracic aorta | Absent | Left lower lobe | TAE with coils and vascular plugs | 10 months | Post-embolization syndrome |
| Abe et al. (5) | 2011 | F/24 | Recurrent hemoptysis | Descending thoracic aorta | Absent | Left lower lobe | TAE with coils | 1 year | Chest pain for 3days |
| Lim et al. | 2009 | M/26 | Recurrent hemoptysis | Descending thoracic aorta | Not mentioned | Left lower lobe | TAE with coils | 6 months | Post-embolization syndrome |
| Muñoz et al. (2) | 2008 | M/29 | Hemoptysis | Abdominal aorta | Normal | Right lower lobe | TAE with coils | 12 months | None |
| Kosutic et al. | 2007 | M/3 mo | Respiratory failure | Descending thoracic aorta (2 separated origins) | Hypoplastic | Right upper lobe | One: TAE with coils The other: spontaneously closed after cardiac catheterization | 6 months | Severe respiratory failure, significant hypercapnia, and multiresistant Pseudomonas infection, which were treated successfully |
| Saida et al. (7) | 2006 | F/41 | Recurrent hemoptysis | Descending thoracic aorta | Absent | Left lower lobe | TAE with coils | 6 years | None |
| Huang et al. | 2005 | F/3 mo | Respiratory distress, cardiac murmur | Descending thoracic aorta | Absent | Left lower lobe | TAE with coils | 6 months | None |
| F/13 | Recurrent hemoptysis | Descending thoracic aorta | Absent | Left lower lobe | TAE with coils | 18 months | Residual arterial supply at 6 months, successfully treated by re-embolization | ||
| Abdulhamid et al. | 2004 | M/13 | Chest pain, hemoptysis | Several arterial collaterals from various levels of left IMA, and the midthoracic right intercostal artery | Not mentioned | Right lung and pericardium | TAE with coils | 18 months | None |
| F/15 | Recurrent hemoptysis | Right innominate a, transverse aortic arch, proximal descending aorta, right IMA | Not mentioned | Right middle and lower lobes | TAE with coils | 18 months | Cough and wheezing for 2 days | ||
| M/7 | Recurrent hemoptysis | Aorta | Not mentioned | Right lung | TAE with coils | 2 years | None | ||
| F/10 | Hemoptysis, choking | Aorta | Not mentioned | Not mentioned | TAE with coils | 9 months | Recurred bleeding due to collateral vessel at 5 months, successfully treated by re-embolization | ||
| Chabbert et al. | 2002 | M/17 | Acute chest pain | Abdominal aorta | Normal | Right lower lobe | TAE with coils | 12 months | Post-embolization syndrome |
| Izillo et al. | 2000 | M/34 | Recurrent hemoptysis | Celiac trunk | Absent | Right lower lobe | TAE with coils | 2 years | Transient and asymptomatic pulmonary infarction |
| Brühlmann et al. (3) | 1998 | M/51 | Massive hemoptysis | Descending thoracic aorta | Normal; small caliber | Left lower lobe | TAE with coils | 10 months | None |
References
1. Kim JH, Kim SS, Ha KS, Bae J, Park Y. Anomalous arterial supply to normal basal segment of the right lower lobe: endovascular treatment with the amplatzer vascular plug. Tuberc Respir Dis (Seoul). 2014; 76:295–298.
2. Muñoz JJ, García JA, Bentabol M, Padín MI, Serrano F. Endovascular treatment of hemoptysis by abnormal systemic pulmonary artery supply. Cardiovasc Intervent Radiol. 2008; 31:427–430.
3. Brühlmann W, Weishaupt D, Goebel N, Imhof E. Therapeutic embolization of a systemic arterialization of lung without sequestration. Eur Radiol. 1998; 8:355–358.
4. Jariwala P, Ramesh G, Sarat Chandra K. Congenital anomalous/aberrant systemic artery to pulmonary venous fistula: closure with vascular plugs & coil embolization. Indian Heart J. 2014; 66:95–103.
5. Abe T, Mori K, Shiigai M, Okura N, Okamoto Y, Saida T, et al. Systemic arterial supply to the normal basal segments of the left lower lobe of the lung--treatment by coil embolization--and a literature review. Cardiovasc Intervent Radiol. 2011; 34:Suppl 2. S117–S121.
6. Do KH, Goo JM, Im JG, Kim KW, Chung JW, Park JH. Systemic arterial supply to the lungs in adults: spiral CT findings. Radiographics. 2001; 21:387–402.
7. Saida T, Ninomiya H, Hojo F, Nakayama M, Yamauchi T, Saida Y. Systemic arterial supply to the normal basal segments of the left lower lobe treated by coil embolization, with long-term follow-up. Radiat Med. 2006; 24:365–368.
8. Pollak JS, Saluja S, Thabet A, Henderson KJ, Denbow N, White RI Jr. Clinical and anatomic outcomes after embolotherapy of pulmonary arteriovenous malformations. J Vasc Interv Radiol. 2006; 17:35–44. quiz 45.
9. Brillet PY, Dumont P, Bouaziz N, Duhamel A, Laurent F, Remy J, et al. Pulmonary arteriovenous malformation treated with embolotherapy: systemic collateral supply at multidetector CT angiography after 2-20-year follow-up. Radiology. 2007; 242:267–276.
10. Anil G, Taneja M, Tan AG. Endovascular treatment of isolated systemic arterial supply to normal lung with coil and glue embolisation. Br J Radiol. 2012; 85:e83–e86.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download