Abstract
Purpose
To evaluate multi-detector CT (MDCT) coronary angiographic findings of coronary-to-pulmonary artery fistula (CPAF).
Materials and Methods
We retrospectively reviewed images of patients with CPAF from the coronary CT angiography (CCTA) database obtained with a 64-channel MDCT between January 2008 and March 2011. We analyzed the CCTA findings for feeding arteries, fistula, association with peripulmonary arterial aneurysms, and the presence of communication between the CPAF and bronchial arteries.
Results
Fifty-five of the 15042 (0.37%) patients were diagnosed with CPAFs. The feeding artery was single (n = 18) or multiple (n = 37). The fistula had a single drainage site (n = 54) or multiple drainage sites (n = 1). The mean diameter of the fistulous opening was 2.7 ± 1.4 mm. A peripulmonary arterial aneurysm was present in 24 (44%) patients. Communication between CPAF and bronchial arteries was present in eight (14.5%) patients.
Coronary artery fistula (CAF) is a rare, abnormal connection between a coronary artery and a great vessel or a cardiac chamber, which bypasses the myocardial capillary bed. Coronary-to-pulmonary artery fistula (CPAF) is a subtype of CAF in which a coronary artery communicates with a pulmonary artery; this can be congenital in origin or acquired (1). Although most patients with CPAFs are asymptomatic and these fistulas are incidentally detected (2), some of these anomalies may cause dyspnea, chest pain, or even sudden death (3).
Multi-detector computed tomography (MDCT) is now widely utilized in the evaluation of known or suspected coronary artery disease (4). MDCT coronary angiography can provide information on the precise anatomy of coronary arteries and associated findings (56). Although there have been a few reports on MDCT evaluation of CPAFs, they are mostly limited in the number of cases evaluated (137); therefore, the detailed anatomy and clinical implications of CPAFs remain to be elucidated. Therefore, the purpose of this study was to evaluate the incidence, clinical findings, and detailed MDCT coronary angiographic findings of CPAFs.
The Institutional Review Board of our institute approved this study and the requirement for informed consent was waived due to the retrospective nature of the study.
Between January 2008 and December 2010, a total of 15042 patients, who underwent contrast-enhanced 64-channel MDCT that included coronary angiography, were included. The patients were referred by a physician for the evaluation of suspected coronary artery disease, or were self-referred due to a personal health concern or for routine checkup. We retrospectively reviewed the radiology reports of CAFs, and 55 consecutive patients (32 males, 23 females; mean age: 53.9 ± 12.3 years; range: 31–78 years) who were diagnosed with CPAFs were included in our study population. Clinical findings, including symptoms, underlying disease, or treatment received, were obtained from the electronic medical records.
All CT scans were performed with a 64-channel dual-source MDCT scanner (Somatom Definition; Siemens Medical Solutions, Forchheim, Germany). For patients with a pre-scanning heart rate of ≥ 65 beats per minute, 100 mg of metoprolol (Betaloc; AstraZeneca, Yuhan, Seoul, Korea) was administered orally 1 h prior to CT examination, unless contraindicated such as in patients with asthma. Sublingual nitroglycerin 0.6 mg (Nitroquick; Ethex, St. Louis, MO, USA) was administered to all subjects for better depiction of the coronary arteries (8). The CT technique has been described elsewhere (9). In brief, according to the coronary CT protocol of our institution, after establishing antecubital intravenous access, 70–90 mL of contrast media (Ultravist 370; Schering, Berlin, Germany) was injected, followed by 50 mL of an 8:2 mixture of normal saline and contrast medium, at a flow rate of 4 mL/s, by means of a dual power injector (Stellant; Medrad, Indianola, PA, USA). The bolus-triggering method was used to determine the beginning of CT acquisition by monitoring the signal density of the contrast medium in the mid-ascending aorta. The CT scan commenced 8 s after the threshold trigger of 150 Hounsfield unit above baseline was reached.
In general, data acquisition was performed in the caudocranial direction during suspended respiration, using a detector collimation of 32 × 0.6 mm, a section collimation of 64 × 0.6 mm with a z-flying focal spot, a gantry rotation time of 330 ms, and a tube voltage of 100 kVp. The pitch was automatically adapted to the heart rate: 0.20 (40 bpm), 0.26 (60 bpm), 0.32 (70 bpm), 0.37 (80 bpm), 0.43 (90 bpm), and 0.50 (100 bpm). Tube current was 320 mA per rotation. Datasets of enhanced images were reconstructed with a 0.6–0.8-mm slice thickness and 0.6–0.8-mm increments, using a B26 soft-tissue reconstruction kernel. The volume CT dose index provided by the CT scanners varied from 8.9 to 55.34 mGy, which corresponded to the effective dose ranging from 1.4 to 8.85 mSv.
Two experienced radiologists (E.A.P. and J.L., with 10 and 3 years of experiences in interpreting cardiac CT scans, respectively) reviewed the transverse axial images and 4-chamber, 2-chamber, and short-axis multi-planar reconstruction images on a workstation installed with three-dimensional reconstruction software (Rapidia; Infinitt, Seoul, Korea). The characteristics of CPAFs, including the number and origin of feeding coronary arteries, relationship with major vessels, course of the fistula itself, the number, size and location of the drainage site of the fistula, association with aneurysmal change, and communication with the bronchial artery, were evaluated. In terms of associated aneurysmal changes, the pre-fistulous dilatation was classified into either an aneurysmal dilatation, which was defined as ≥ 3 mm to < 6 mm in diameter, or a saccular aneurysm, which was defined as ≥ 6 mm in diameter.
Among the 15042 patients who underwent MDCT coronary angiography examinations during the period, a total of 55 (0.37%) patients were diagnosed with CPAFs based on CT findings.
Clinical symptoms and underlying diseases are provided in Table 1. Most of the patients (65%) were asymptomatic and CPAFs were detected during a routine checkup. Common symptoms included typical chest pain (17%), atypical chest pain (11%), and dyspnea (7%). Of the 55 patients, 12 (22%) patients had concomitant diseases: valvular heart disease in four (7%) patients, coronary artery disease in three (5%) patients, aortic aneurysm in three (5%) patients, atrial septal defect in one (2%) patient, and hypertrophic cardiomyopathy in one (2%) patient.
Surgical obliteration of CPAFs was performed in two patients during mitral valvuloplasty and repair of the atrial septal defect, respectively. Coiling of CPAFs was attempted in three patients who were free of coronary artery disease, and it was successful in one patient. Coiling of CPAF was unsuccessful in the other two patients because of unfavorable anatomy of the coronary artery and CPAF. These patients showed myocardial ischemia symptom and were subjected to treatment. As a result, three (5%) patients underwent surgical treatment or coiling of CPAF and the remaining 52 (94.5%) patients were subjected to conservative management, including observation, or medication such as beta-blockers and calcium channel blockers for their underlying cardiac diseases. During the follow-up period, two (4%) patients died because of pneumonia and the remaining 53 (96%) patients did not present remarkable symptoms. There was no mortality associated with the cardiac origin.
The origin of feeding arteries of the CPAF was singular in 18 (33%) patients and multiple in 37 (67%) patients. In the patients with a single arterial origin, the right conus artery was involved in four cases and the left coronary artery (LCA) was involved in the remaining 14 cases: there were 11 cases with an origin in the left anterior descending artery (LAD) and three cases with a left main (LM) origin. In all patients with multiple arterial origins, both right and left coronary arteries contributed to the CPAF (Fig. 1). On the right side, feeding arteries originated from the right conus artery in all cases. On the left side, various combinations of LM, LAD, and left circumflex artery (LCX) were found; there were 22 cases with feeding arteries arising from the LAD, five cases with feeding arteries arising from the LM, seven cases with feeding arteries arising from both the LM and the LAD, and three cases with feeding arteries arising from both the LAD and the LCX. The feeding arteries arising from the septal branch of the LM and the diagonal branch were considered to be LM feeders and LAD feeders, respectively (Table 2).
Most patients (54 of 55, 98%) showed a single drainage site of the fistula, at the left sinus of the main pulmonary artery (MPA). In one patient, the CPAF had multiple fistulous drainage sites that were located in both the left and right sinuses of the MPA (Fig. 2). The mean diameter of the fistulous opening was 2.7 ± 1.4 mm (range, 1.0–8.0 mm). In approximately half of the patients (24 of 55, 44%), dilatation of the CPAF was present before the fistulous drainage site in the MPA: an aneurysmal dilatation (mean, 4.4 ± 0.9 mm, range, 3.0–5.9 mm) in 17 (71%) patients or a saccular aneurysm (mean, 10.3 ± 7.3 mm, range, 6.2–26.0 mm) in seven (29%) patients.
In addition, communication between the CPAF and bronchial arteries was noted in eight (14.5%) patients (Fig. 3). The origin of coronary arteries communicating with bronchial arteries was from the LM in two patients, the LAD in three patients, both the LAD and the LM in two patients, and both the conus artery and the LM in one patient. The origin of the bronchial arteries was observed in seven patients, and all bronchial arteries budded from the descending thoracic aorta. The mean diameter of the fistulous opening was 3.5 ± 2.2 mm (range, 1.0–5.5 mm). Seven patients showed peripulmonary dilatation: aneurysmal dilatation was seen in six patients and a saccular aneurysm was seen in one patient.
Our study showed that MDCT coronary angiography could provide details on the morphologic features of CPAFs, including the origin of the feeder arteries, the course of the involved vessels, information about the drainage site of the fistula, and the presence and nature of pre-fistulous dilatation. A subtype of CPAFs, formation of an additional fistula with the bronchial artery, could also be delineated, with a comprehensive overview of the complex anatomy.
CPAFs can have a congenital or acquired cause. Congenital CPAF is thought to be associated with the development of coronary arteries. During 33 days to 36 days of the embryonic period, a primitive vascular network develops around the aorta and pulmonary artery; coronary arteries are formed by an ingrowth from this precursor (10). When this process is deranged, coronary arteries make an abnormal ingrowth into the pulmonary artery, and CPAFs may occur with enlargement due to the increase in systemic blood pressure (Fig. 4) (11). With respect to acquired CPAFs, coronary artery bypass surgery is known to be the most frequent cause, while percutaneous intervention in the coronary arteries is thought to be another possible cause (12). Although the mechanism of development of acquired CPAFs remains unclear, it has been suggested that specific organizing proteins may become activated after surgery and promote neovascularization (13). Our cases are also assumed to be those of congenital CPAFs because all patients have neither surgical history nor underlying disease promoting neovascularization.
The incidence of CPAFs has been reported to be 0.1–0.2% in patients undergoing cardiac catheterization (1415), while it was 0.37% in this study. Our result is quite similar to that in another CT study by Kim et al. (16), who found 17 (0.32%) cases upon reviewing 5372 patients. This discrepancy with cardiac catheterization could have resulted from the difference in the modality used. In our study, 67% of CPAFs originated from both the right and left coronary arteries, which is in accordance with previous studies using CT (53–59%) (16171819). The remaining 33% of CPAFs in our study originated from a single coronary artery; 25% of CPAFs originated from the LCA and 8% of CPAFs originated from the RCA. This predominance of single artery-origin CPAFs involving the LCA is also in accordance with the results of previous studies, where it has been reported to account for 29% to 42% (1718) of such cases.
Identification of the drainage site of a CPAF is thought to be clinically more important for surgical closure than identification of the origin. In the present study, CPAFs in most of the patients (54 of 55, 98%) drained into a single site at the left sinus of the MPA, while the CPAF in only one patient showed multiple drainage sites at both the left and right sinuses of the MPA. This finding is generally in agreement with the results of previous studies where 82% to 88% of patients demonstrated a drainage site on the left lateral aspect of the MPA (1617).
Remarkably, approximately half of the CPAFs in the present study (24 of 55, 44%) demonstrated pre-fistulous dilatation. Among them, seven (29%) patients had a saccular aneurysm, defined as ≥ 6 mm in diameter. This result was consistent with previous studies that also delineated focal or multifocal dilatation of coronary artery branches around the drainage site of CPAFs in 15–35% of cases (16171820). The mechanism of this aneurysmal change in the CPAF is thought to involve increased blood flow in the coronary artery, as in CAFs (2122).
It is believed that the clinical presentation of CAFs depends on the extent of the left-to-right shunt (23). Many cases of CPAF are asymptomatic, as the amount of shunting is not significant (24). However, a large difference in pressure across the shunt may cause the fistula to become tortuous and dilated, which can result in aneurysmal changes with the risk of rupture (112425). In addition, coronary steal could occur due to the increased size of the distal portion of the fistula (18). Symptoms of the patients in this study varied from none to chest pain, including atypical chest pain and dyspnea. Approximately 65% (36 of 55) of the patients were asymptomatic, while the remaining 35% (19 of 55) of the patients showed cardiopulmonary symptoms. This finding was in accordance with a previous report, which stated that a major proportion of adult patients with CAFs are usually asymptomatic (23). In CAFs, symptoms are thought to be due to coronary steal (26), and this may also be the case in CPAFs.
Generally, treatment of congenital CPAF is not recommended when it is asymptomatic, because its hemodynamic effect is not significant (27). However, closure of the fistula is indicated in patients with myocardial ischemia, congestive heart failure, and a large left-to-right shunt (28). Surgery has been performed for the management of CPAFs, and recently, endovascular treatment using stent grafts or coils has been performed. Transcatheter coil embolization causes the blood flow to slow down, which results in thrombosis in the fistula (12). It has been reported that the results of surgery and transcatheter embolization are similar with respect to early effectiveness as well as morbidity and mortality, although most of these results were obtained from cases with CAFs (23).
There were eight cases (14.5%) of CPAFs that demonstrated a communication with bronchial arteries. Although the incidence is not known due to lack of reports, it is expected to be significantly lower than that of other types of “less-developed” fistulas such as CPAF and coronary-to-bronchial artery fistula. The incidence was higher than expected, but the results of a previous study of 99 patients with CPAF support our data, and it shows that ten (10%) patients were complicated with the communication with extracardiac arteries. It had been postulated that CPAFs with bronchial communication could be the result of a congenital or acquired cause. In our study, all eight patients did not have a history of chest trauma, surgery or pulmonary parenchymal diseases. CPAFs with bronchial communication may also cause myocardial ischemia symptom due to coronary steal syndrome (29). In our study, two patients presented with chest pain and one patient was diagnosed as having angina pectoris. Another possible clinical significance lies in the management. When bronchial artery embolization is performed, embolic material deployed in the bronchial arteries can migrate into the coronary arteries and cause fatal results such as myocardial infarction (30). Therefore, it is recommended that bronchial artery embolization should be performed after a delicate review of courses of bronchial arteries to exclude the possibility of a fistula between bronchial arteries and the coronary artery.
There are several limitations to our study. First, this study was performed in a single institution, and its small sample size is a limitation. Second, this is a retrospective study based on electronic medical records and an imaging database whose target population consisted of patients who were suspected of having coronary artery disease or were concerned about their health. This can create a bias, as individuals who visit the hospital due to a health concern may have more disease than healthy people in the community. Therefore, the incidence of CPAF reported in the present study could be higher than the actual incidence. Third, the reference standard was not established in some of our cases as coronary angiography was not performed in those patients. Lastly, there could be limitations related to the CT modality. Although CCTA provided an excellent and comprehensive overview of the fistula, allowing detailed description of tortuous, small vascular branches, very fine vessels may have been overlooked due to the limitation of the axial resolution of CT. In addition, low-pressure circulation inside the CPAF could have caused a decrease in the blood flow, which could result in reduction of the size of the fistula (27).
In conclusion, the incidence of CPAF as detected by MDCT is higher than that previously reported with the use of invasive coronary angiography. CPAF has a single feeding vessel or multiple feeding vessels from coronary arteries, and in most cases, it has a single drainage site at the left sinus of the MPA. Pre-fistulous aneurysmal dilatation is a common feature of CPAFs. CPAFs also show communication with the bronchial artery in some instances. MDCT coronary angiography can provide a comprehensive analysis of CPAFs and it has the advantage of being non-invasive over conventional coronary angiography. Knowledge of the CT morphology of CPAFs with respect to the number, size and location of drainage sites of the fistula and associated aneurysms helps in presurgical or preinterventional planning.
Figures and Tables
Fig. 1
Coronary-to-pulmonary artery fistula (CPAF) with multiple feeding arteries in a 59-year-old man.
A. The tortuous vessels of CPAF (arrowhead) pass anterior to the pulmonary artery, forming a network and focal aneurysmal sac (black arrow) before they enter the main pulmonary artery.
B, C. The multiple feeding arteries of the CPAF arise from (B) the right coronary artery (white arrow) and (C) the proximal left anterior descending artery (yellow arrow).
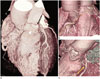
Fig. 2
Drainage sites of coronary-to-pulmonary artery fistulae (CPAFs).
A. A case of a 63-year-old man. The single drainage site is located in the left sinus of the main pulmonary artery (red arrow).
B. A case of a 70-year-old man. The CPAF drained into three different points in the left and right sinuses of the main pulmonary artery (yellow arrows).
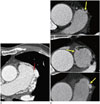
Fig. 3
Coronary-to-pulmonary artery fistula (CPAF) communicating with bronchial arteries in a 63-year-old man. The bronchial artery originates from the proximal descending thoracic aorta (black arrow). It runs under the left pulmonary artery (red arrows) and communicates with the abnormal serpentine vessels that lead to the formation of CPAF.
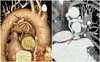
Fig. 4
Development of coronary-to-pulmonary artery fistula (CPAF), illustrated based on references (910).
A. Peritruncal vascular network formation around the primitive aorta and pulmonary artery.
B. Development of CPAF as a result of abnormal vasculogenesis in the left sinus of the main pulmonary artery (MPA) with accompanying aneurysmal
dilatation.
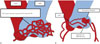
Table 1
The Characteristics of the Patients with Coronary-to-Pulmonary Artery Fistula

Table 2
Number and Origin of Feeding Arteries of Coronary-to-Pulmonary Artery Fistula

References
1. Demirelli S, Tas MH, Simsek Z, Duman H, Ipek E. Coronaryto-pulmonary artery fistula and concomitant acute coronary syndrome: two cases. J Case Rep. 2014; 4:56–59.
2. Vaidyanathan KR, Theodore SA, Sankar MN, Cherian KM. Coronary artery to pulmonary artery fistula with dual origin--embryological, clinical and surgical significance. Eur J Cardiothorac Surg. 2007; 31:318–319.
3. Gandy KL, Rebeiz AG, Wang A, Jaggers JJ. Left main coronary artery-to-pulmonary artery fistula with severe aneurysmal dilatation. Ann Thorac Surg. 2004; 77:1081–1083.
4. Vanhoenacker PK, Heijenbrok-Kal MH, Van Heste R, Decramer I, Van Hoe LR, Wijns W, et al. Diagnostic performance of multidetector CT angiography for assessment of coronary artery disease: meta-analysis. Radiology. 2007; 244:419–428.
5. Dodd JD, Ferencik M, Liberthson RR, Cury RC, Hoffmann U, Brady TJ, et al. Congenital anomalies of coronary artery origin in adults: 64-MDCT appearance. AJR Am J Roentgenol. 2007; 188:W138–W146.
6. Haller S, Kaiser C, Buser P, Bongartz G, Bremerich J. Coronary artery imaging with contrast-enhanced MDCT: extracardiac findings. AJR Am J Roentgenol. 2006; 187:105–110.
7. Ohkura K, Yamashita K, Terada H, Washiyama N, Akuzawa S. Congenital systemic and coronary-to-pulmonary artery fistulas. Ann Thorac Cardiovasc Surg. 2010; 16:203–206.
8. Takx RA, Suchá D, Park J, Leiner T, Hoffmann U. Sublingual nitroglycerin administration in coronary computed tomography angiography: a systematic review. Eur Radiol. 2015; 25:3536–3542.
9. Park EA, Lee W, Na SH, Chung JW, Park JH. Left ventricular fat deposition on CT in patients without proven myocardial disease. Int J Cardiovasc Imaging. 2013; 29:Suppl 1. 37–45.
10. de Oliveira, da Silva, Mandarimde-Lacerda CA. Origin and development of the coronary arteries. Int J Morphol. 2009; 27:891–898.
11. Kazuno K, Akasaka N, Kiyokawa K, Sasajima T. Ruptured aneurysm of coronary artery-to-pulmonary artery fistula. Asian Cardiovasc Thorac Ann. 2012; 20:324–326.
12. Chang DS, Lee MH, Lee HY, Barack BM. MDCT of left anterior descending coronary artery to main pulmonary artery fistula. AJR Am J Roentgenol. 2005; 185:1258–1260.
13. Angelini P. Coronary-to-pulmonary fistulae: what are they? What are their causes? What are their functional consequences? Tex Heart Inst J. 2000; 27:327–329.
14. Said SA, Landman GH. Coronary-pulmonary fistula: long-term follow-up in operated and non-operated patients. Int J Cardiol. 1990; 27:203–210.
15. Urrutia-S CO, Falaschi G, Ott DA, Cooley DA. Surgical management of 56 patients with congenital coronary artery fistulas. Ann Thorac Surg. 1983; 35:300–307.
16. Kim MS, Jung JI, Chun HJ. Coronary to pulmonary artery fistula: morphologic features at multidetector CT. Int J Cardiovasc Imaging. 2010; 26:Suppl 2. 273–280.
17. Lim JJ, Jung JI, Lee BY, Lee HG. Prevalence and types of coronary artery fistulas detected with coronary CT angiography. AJR Am J Roentgenol. 2014; 203:W237–W243.
18. Saboo SS, Juan YH, Khandelwal A, George E, Steigner ML, Landzberg M, et al. MDCT of congenital coronary artery fistulas. AJR Am J Roentgenol. 2014; 203:W244–W252.
19. Zhang LJ, Zhou CS, Wang Y, Jin Z, Yu W, Zhang Z, et al. Prevalence and types of coronary to pulmonary artery fistula in a Chinese population at dual-source CT coronary angiography. Acta Radiol. 2014; 55:1031–1039.
20. Erol C, Seker M. Coronary artery anomalies: the prevalence of origination, course, and termination anomalies of coronary arteries detected by 64-detector computed tomography coronary angiography. J Comput Assist Tomogr. 2011; 35:618–624.
21. Hoendermis ES, Waterbolk TW, Willems TP, Zijlstra F. Large common left and right coronary artery to coronary sinus fistula. Interact Cardiovasc Thorac Surg. 2006; 5:788–789.
22. van Geuns RJM, Cademartiri F. Anatomy of the coronary arteries and veins in CT imaging. In : Schoepf UJ, editor. CT of the Heart. Totowa, NJ: Humana Press;2005. p. 219–227. .
23. Gowda RM, Vasavada BC, Khan IA. Coronary artery fistulas: clinical and therapeutic considerations. Int J Cardiol. 2006; 107:7–10.
24. Shabestari AA, Akhlaghpoor S, Fatehi M. Findings of bilateral coronary to pulmonary artery fistula in 64-multislice computed tomographic angiography: correlation with catheter angiography. J Comput Assist Tomogr. 2008; 32:271–273.
25. Shriki JE, Shinbane JS, Rashid MA, Hindoyan A, Withey JG, DeFrance A, et al. Identifying, characterizing, and classifying congenital anomalies of the coronary arteries. Radiographics. 2012; 32:453–468.
26. Rittenhouse EA, Doty DB, Ehrenhaft JL. Congenital coronary artery- cardiac chamber fistula. Review of operative management. Ann Thorac Surg. 1975; 20:468–485.
27. Tomasian A, Lell M, Currier J, Rahman J, Krishnam MS. Coronary artery to pulmonary artery fistulae with multiple aneurysms: radiological features on dual-source 64-slice CT angiography. Br J Radiol. 2008; 81:e218–e220.
28. Lai MC, Chen WJ, Chiang CW, Ko YL. An unusual case of dual coronary artery fistulas to main pulmonary artery. Chang Gung Med J. 2002; 25:51–55.
29. Härle T, Kronberg K, Elsässer A. Coronary artery fistula with myocardial infarction due to steal syndrome. Clin Res Cardiol. 2012; 101:313–315.
30. Qiu MJ, Dong DJ. Myocardial infarction following bronchial artery embolization for hemoptysis. Chin Med J (Engl). 2013; 126:997.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download