Abstract
A simple renal cyst usually remains asymptomatic and requires no invasive treatment. Occasionally, however, some cysts may cause pain, hematuria, hypertension, or obstruction of the collecting system. We describe a case of a 50-year-old man who presented with hydronephrosis caused by an ipsilateral simple renal cyst without any stone or significant mass in the urinary system. The patient was eventually treated successfully with sclerotherapy.
A simple renal cyst is defined as a benign, fluid-filled, non-neoplastic lesion. In most cases, the cysts remain asymptomatic and usually require no invasive treatment (1). Occasionally, however, some cysts may cause pain, hematuria, hypertension, or obstruction of the collecting system (2). We report an unusual case of a patient with hydronephrosis due to a simple renal cyst that was treated successfully with percutaneous sclero-therapy using ethanol.
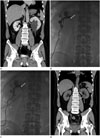
A 50-year-old man presented to the emergency department with sudden onset of left flank pain. For about a decade the patient has had a history of ureteral stones. During a physical examination, no abnormality was found except for left costovertebral angle tenderness. All laboratory findings were within the normal range, including blood and urine tests. Contrast-enhanced computed tomography (CT) was performed to exclude a recurrent ureteral stone. CT showed hydronephrosis in the left urinary system without any obstructive lesion such as a stone or significant mass. In addition, there was a 5.8 cm renal cortical cyst without calcification or an enhancing solid nodule that was protruding into the renal sinus at the lower pole of the left kidney (Fig. 1A). This simple renal cyst was the only suspected cause of left-sided hydronephrosis. Percutaneous nephrostomy (PCN) was performed to decompress the left-sided hydronephrosis, relieving the symptom. A few days later, we performed an antegrade pyelogram (AGP) through the PCN catheter to evaluate an accurate level of obstruction. On the AGP, contrast media only filled the dilated pelvocalyceal system that was compressed by the renal cyst, and did not pass well through the level of the ureteropelvic junction (Fig. 1B). We performed percutaneous drainage of the renal cyst. After aspiration of the cyst through the drainage catheter, contrast media was able to pass through the ureteropelvic junction (Fig. 1C) without complication. Although about 40 mL of serous fluid was aspirated from the renal cyst, we decided to perform sclerotherapy because of the fact that the simple renal cyst was confirmed to be the cause of the urinary obstruction on the AGP. A cystogram was obtained by using contrast media to evaluate the presence of extravasation or the communication of the cyst with the collecting system. After demonstration of absence of extravasation or communication with the collecting system, all of the volume was aspirated. Then single session sclerotherapy was performed with 20 mL of absolute ethanol via the drainage catheter. Ethanol was left in the cavity for 60 min. During this period, the patient was placed in at least three of supine, prone and both lateral decubitus positions, each for 15 min to allow contact of ethanol with the entire wall of the cysts. At the end of the procedure, ethanol was aspirated and the catheter was withdrawn without additional saline irrigation. The procedure was completed without any complications. After 1 month, a follow-up CT revealed no hydronephrosis in the left kidney and the size of the renal cyst had decreased to 2 cm (Fig. 1D).
Renal cysts are common abnormalities and they can be found incidentally in most elderly patients. The incidence of renal cysts is as high as 50% in the population during the 6th decade of life or more, and the size of the cysts also increase with age (1). Simple renal cysts are usually clinically silent and treatment is not required; however, they may be associated with flank pain, hypertension, hematuria, infection, and obstruction of the collecting system (2). Until now, a few cases of obstruction of the collecting system caused by a simple renal cyst have been reported in the literature (3). But, obstruction of the collecting system caused by parapelvic cysts have been reported many times compared with obstruction of the collecting system caused by renal cysts (4). The origins of renal cysts and parapelvic cysts are different. Renal cysts are more common in older age groups, and thus, they are believed to be acquired lesions. Although the pathogenesis is not well known, it is believed that they originate from the diverticulae of the distal convoluted or collecting tubules (5). On the other hand, parapelvic cysts arise from lymphatic tissues in the renal sinus and they are thought to develop from embryologic rests (4). Due to the difference in their origin and location, renal cysts and parapelvic cysts are considerably different in terms of the incidence rates of obstruction of the collecting system.
Evans and Coughlin (3) insisted that the size of the renal cyst has little relation to the degree of calyceal obstruction. Rather it was the position of the cyst in relation to the hilum or calyceal infundibulum and its ability to expand causing compression of the surrounding tissue that determined the degree of the obstruction. In addition, according to another report, two requirements should be met for a renal cyst to cause obstruction: 1) a central position within or near the hilum or in its extension along the infundibular origin and 2) sufficient turgor to produce pressure greater than that in the pelvic infundibula (6). In our case, the renal cortical cyst protruded into the renal hilum. Although its size was not large, it is presumed that the position of the cyst related to the hilum is the main cause of urinary obstruction. Therefore, it is important to check the location of the renal cyst under the circumstance of pelvocalyceal obstruction without a definite cause of urinary obstruction, except for a renal cyst.
In asymptomatic cases, renal cysts may be left untreated. However, when the adjacent pelvocalyceal system is compressed by the renal cyst as in our case, a therapeutic measure must be used in order to decompress the hydronephrosis. Treatment options for symptomatic renal cysts include surgical or laparoscopic excision, simple percutaneous drainage and percutaneous drainage followed by instillation of a sclerosant. However, surgery is invasive and it requires general anesthesia with the accompanying operative morbidity and complications. Thus, these techniques have been replaced by minimally invasive approaches that are based on a percutaneous aspiration with or without the use of a sclerosing agent to destroy the epithelial cells of the cyst wall (7). Ethanol is the most commonly used sclerosant for cyst ablation; sclerotherapy using ethanol and drainage catheter for a renal cyst is a safe, inexpensive, and effective treatment option in terms of symptom improvement (8). We used 20 mL of ethanol, and the early result in this case was adequate enough to relieve the ureteral obstruction although the cyst did not disappear completely.
To perform sclerotherapy, there are several points to note. Before the instillation of sclerosing agents, check the presence of extravasation of contrast media or the connection with the renal collecting system through the cystogram to prevent the peritonitis, retroperitoneal fibrosis or stricture of the collecting system. After complete aspiration of the contrast media in the cyst, ethanol in a quantity of 20–50% of the aspirated volume should be injected. However, in order to minimize the possible side effect of a huge cyst the maximal amount of ethanol is limited to 100 mL. It is important to roll the position of the patient into supine, prone, and lateral decubitus at 10- to 15-min intervals to allow contact of ethanol with all surfaces of the cyst.
In conclusion, our case demonstrates that hydronephrosis can be caused by a simple renal cyst without any accompanying lesion such as a stone or a significant mass in the urinary system. In the presence of compression of the adjacent pelvocalyceal system, percutaneous sclerotherapy using ethanol may serve as a treatment option for a simple renal cyst.
Figures and Tables
Fig. 1
Sclerotherapy for a simple renal cyst causing hydronephrosis in a 50-year-old man.
A. Coronal reformatted contrast-enhanced CT scan shows a renal cortical cyst located at the lower pole of the left kidney (arrows). Note that there is a dilated pelvocalyceal system in the left kidney with perinephric infiltration suggestive of hydronephrosis.
B. Antegrade pyelogram through the percutaneous nephrostomy catheter. Contrast media does not pass well through the level of ureteropelvic junction (arrow).
C. Antegrade pyelogram after aspiration of the renal cyst through the percutaneous drainage catheter. Contrast media passes well through the ureteropelvic junction (arrow).
D. Follow-up CT scan obtained one-month later shows no hydronephrosis in the left kidney and reduction of the size of the renal cyst (arrows).
References
1. Terada N, Arai Y, Kinukawa N, Terai A. The 10-year natural history of simple renal cysts. Urology. 2008; 71:7–11. discussion 11-2.
2. Caglioti A, Esposito C, Fuiano G, Buzio C, Postorino M, Rampino T, et al. Prevalence of symptoms in patients with simple renal cysts. BMJ. 1993; 306:430–431.
3. Evans AT, Coughlin JP. Urinary obstruction due to renal cysts. J Urol. 1970; 103:277–280.
4. Hidalgo H, Dunnick NR, Rosenberg ER, Ram PC, Korobkin M. Parapelvic cysts: appearance on CT and sonography. AJR Am J Roentgenol. 1982; 138:667–671.
5. Baert L, Steg A. Is the diverticulum of the distal and collecting tubules a preliminary stage of the simple cyst in the adult? J Urol. 1977; 118:707–710.
6. Hinman F Jr. Obstructive renal cysts. J Urol. 1978; 119:681–683.
7. Akinci D, Akhan O, Ozmen M, Gumus B, Ozkan O, Karcaaltincaba M, et al. Long-term results of single-session percutaneous drainage and ethanol sclerotherapy in simple renal cysts. Eur J Radiol. 2005; 54:298–302.
8. Bean WJ. Renal cysts: treatment with alcohol. Radiology. 1981; 138:329–331.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download