Abstract
Anastomosing hemangioma (AH) is a rare and benign vascular neoplasm that is regarded as a morphological variant of capillary hemangioma. AH has been encountered primarily in the kidney. To our knowledge, para-aortic involvement of AH has not been reported previously. Here, we report a case of slowly progressing AH involving the left para-aortic region in a 72-year-old woman with a history of breast cancer surgery. A contrast-enhanced, dynamic abdominal CT scan revealed that the lesion had peripheral enhancement with slow centripetal fashion, which is an enhancement pattern similar to that of hepatic hemangioma.
Primary vascular tumors of the retroperitoneum, including hemangioma, hemangiopericytoma and angiosarcoma, are very rare neoplasms (1). Anastomosing hemangioma (AH) is a subtype of capillary hemangioma that is characterized histologically by an anastomosing pattern of capillary sized vascular channels that are reminiscent of splenic sinusoids (2). Although AH is known to have a predilection for the kidney, it can also sporadically involve the ovary, liver, gastrointestinal tract, adrenal gland and urinary bladder (3456). To the best of our knowledge, AH involving the para-aortic region has not been reported. Reported cases of AH involving less common sites described CT findings, but did not focus on these imaging findings (456). Thus, we present CT findings of AH in the para-aortic region with a brief review of the relevant literature.
A 72-year-old woman with a history of invasive ductal carcinoma of the breast underwent a right partial mastectomy at our institution four years ago. There was no clinical evidence of tumor recurrence until three years after surgery. However, a low-density lesion measuring 3 cm at hepatic segment 4 was incidentally detected on a chest CT scan in the course of periodic surveillance for metastatic disease. Thus, a contrast-enhanced dynamic abdominal CT scan was recommended for detailed analysis of the lesion. The low density hepatic lesion showed no contrast enhancement in the three phases of dynamic CT, and no significant interval change was observed on follow-up CT examination. Although the hepatic lesion was not confirmed histologically, it was considered to be a benign lesion, like a cyst. The patient had no symptoms at that time, and all laboratory data including urinalysis were within normal range.
Meanwhile, the contrast-enhanced dynamic CT scan revealed a mass measuring about 4 × 3.2 cm in the left para-aortic region. This mass demonstrated progressive centripetal enhancement from the periphery, and a central, heterogeneous low density with a speckled appearance (Fig. 1A-C). The differential diagnosis included paraganglioma and hyaline vascular Castleman disease. At that time, we retrospectively reviewed the positron emission tomography (PET)/CT scan that was obtained four years previously, and detected a 2.7 cm mass in the left para-aortic region on the CT portion of the PET/CT (Fig. 1D). The mass did not show fluorodeoxyglucose uptake, and it was missed during our initial interpretation of the PET/CT. Therefore, the patient underwent excision of the left para-aortic mass to exclude the possibility of metastasis.
Upon surgical resection, a well-capsulated round mass, measuring approximately 4 cm in diameter, was found. Microscopically, the tumor demonstrated proliferation of irregular vascular channels lined by hobnail endothelial cells (Fig. 1E). Atypia and mitosis were not noted. Immunohistochemical analysis showed that the tumor cells were diffusely positive for CD31, and the stromal cells were positive for smooth muscle actin. Ki-67 expression was found in approximately 1% of the tumor cells, indicating a lower proliferation index for the tumor. These findings were consistent with AH. The patient was followed up for one year after surgery, and no tumor recurrence was noted.
Hemangiomas are conventionally classified as either cavernous or capillary histological subtypes. Most previously reported liver, kidney and ovary hemangiomas are classified as cavernous (3). Montgomery and Epstein (2) presented a new variant of capillary renal hemangiomas that had distinctive overlapping features of both sinusoidal and hobnail hemangiomas of the skin and soft tissues. They termed the tumor as AH.
AH has been more commonly found in the genitourinary system, particularly in kidneys (2367). Rare cases of AH in other locations, including the testes, ovaries, thigh, abdominal wall, adrenal gland, liver, gastrointestinal tract, and urinary bladder have been reported (2345). Generally, most renal lesions occur unilaterally, and range in size from 0.6 to 5.0 cm (average: 2.1 cm) (6). Although the reported clinical manifestations of renal AH were hematuria and flank pain, a significant proportion of cases were asymptomatic (3). AH showed no significant sex predilection, and many cases were associated with end stage renal disease (6). The literature describes a good prognosis for AH after surgical treatment (6). There are no reported instances of recurrence, metastasis or death from AH. Zhang et al. (7) reported a case of AH that progressed slowly over a four-year observation period. The present case also exhibited slow growth over a three year period.
There is limited imaging data available for AH, and when it is available, it is typically described as having non-specific features. In addition, imaging may vary according to the location and size of the tumor (3). The kidney is the most common site for AH, where CT of the large lesions usually show them to be well-demarcated, heterogeneous solid masses. These large lesions can mimic renal cell carcinoma or other malignancies (36). In contrast, smaller AH lesions may not be visible (3). A similar heterogeneous appearance with peripheral enhancement was dem-onstrated in CT of the adrenal gland (4). AH in the urinary bladder was observed as a sharply defined, small mass with marked homogeneous enhancement (5). Tao et al. (6) reported that renal AH lesions exhibited persistent centripetal enhancement on contrast-enhanced dynamic CT, similar to the enhancement pattern observed in the present case.
Primary retroperitoneal tumors are relatively rare and account for only 0.1% to 0.2% of all malignancies in the body. However, 70% to 80% of them are malignant in nature (1). Among these, retroperitoneal hemangioma is extremely rare in adulthood, and has been confirmed in only 1% to 3% of all retroperitoneal tumors (8). The most common type of previously reported retroperitoneal hemangioma is the cavernous type (9). A few cases of retroperitoneal cavernous hemangioma were described as a cystic masses with minor or poor enhancement on enhanced CT (8). However, a case of retroperitoneal hemangioma with a capillary component exhibited a similar enhancement pattern to that of hepatic hemangioma (9). The present case also exhibited this enhancement pattern, which is expected because AH is a capillary subtype of hemangioma.
The differential diagnosis of a hypervascular tumor in the retroperitoneal space includes paraganglioma, solitary fibrous tumor, undifferentiated pleomorphic sarcoma, hyaline vascular Castleman disease, and other forms of sarcoma (1). Paraganglioma, solitary fibrous tumor and undifferentiated pleomorphic sarcoma tend to be larger than AH tumors and have more necrosis and calcifications (110). Hyaline vascular Castleman disease exhibits more homogeneous enhancement than the present case of AH tumor (10).
In conclusion, we describe a hemangioma variant in the para-aortic region that shows an anastomosing pattern of vascular channels on pathological examination. The incidence of this variant is very low. However, AH could be included in the differential diagnosis because of the presence of a slowly progressing, heterogeneous mass in the para-aortic region, which had a CT enhancement pattern resembling a typical hepatic hemangioma.
Figures and Tables
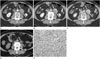 | Fig. 1Anastomosing hemangioma involving the para-aortic region in a 72-year-old woman.
A-C. Contrast-enhanced dynamic CT images show a mass (arrows) measuring about 4 cm in the left para-aortic region. During the arterial phase (A), the mass shows peripheral enhancement. The mass shows slow progressive centripetal enhancement during the portal venous (B) and delayed phase (C) with central heterogeneous low density.
D. The CT portion of a PET/CT image obtained three years before the present CT scan (Fig. 1) shows an oval mass (arrows) measuring about 2.7 cm in the left para-aortic region, which was missed on initial interpretation. The mass did not show FDG uptake on the PET image (not shown).
E. Photomicrograph (H&E staining, × 200) of the para-aortic mass shows proliferation of irregular vascular channels lined by hobnail endothelial cells. Cellular atypia and mitosis are not noted.
FDG = fluorodeoxyglucose, PET = positron emission tomography
|
References
1. Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH Jr, Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics. 2011; 31:949–976.
2. Montgomery E, Epstein JI. Anastomosing hemangioma of the genitourinary tract: a lesion mimicking angiosarcoma. Am J Surg Pathol. 2009; 33:1364–1369.
3. Kryvenko ON, Gupta NS, Meier FA, Lee MW, Epstein JI. Anastomosing hemangioma of the genitourinary system: eight cases in the kidney and ovary with immunohistochemical and ultrastructural analysis. Am J Clin Pathol. 2011; 136:450–457.
4. Ross M, Polcari A, Picken M, Sankary H, Milner J. Anastomosing hemangioma arising from the adrenal gland. Urology. 2012; 80:e27–e28.
5. Jin LU, Liu J, Li Y, Sun S, Mao X, Yang S, et al. Anastomosing hemangioma: the first case report in the bladder. Mol Clin Oncol. 2016; 4:310–312.
6. Tao LL, Dai Y, Yin W, Chen J. A case report of a renal anastomosing hemangioma and a literature review: an unusual variant histologically mimicking angiosarcoma. Diagn Pathol. 2014; 9:159.
7. Zhang W, Wang Q, Liu YL, Yu WJ, Liu Y, Zhao H, et al. Anastomosing hemangioma arising from the kidney: a case of slow progression in four years and review of literature. Int J Clin Exp Pathol. 2015; 8:2208–2213.
8. Hanaoka M, Hashimoto M, Sasaki K, Matsuda M, Fujii T, Ohashi K, et al. Retroperitoneal cavernous hemangioma resected by a pylorus preserving pancreaticoduodenectomy. World J Gastroenterol. 2013; 19:4624–4629.
9. Godar M, Yuan Q, Shakya R, Xia Y, Zhang P. Mixed capillary venous retroperitoneal hemangioma. Case Rep Radiol. 2013; 2013:258352.
10. Surabhi VR, Menias C, Prasad SR, Patel AH, Nagar A, Dalrymple NC. Neoplastic and non-neoplastic proliferative disorders of the perirenal space: cross-sectional imaging findings. Radiographics. 2008; 28:1005–1017.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download