Abstract
Purpose
To propose the imaging classification of true tracheal bronchus (TTB) on multi-detector computed tomography (MDCT), and to evaluate its anatomical relationship with surrounding structures.
Materials and Methods
This study included 44 patients who were diagnosed with TTB on MDCT for 6 years. We classified TTB into five types, based on the existence of the right upper lobe bronchus originating from the right main bronchus and the number of segmental bronchi of TTB. We analyzed the site of origin and the running direction of TTB based on its anatomical relationship with surrounding structures and some ancillary findings.
Results
The imaging classification of TTB included Type I (47.7%), Type II (13.6%), Type III (11.4%), Type IV (25.0%), and Type V (2.0%). According to the site of origin of TTB, below the aortic arch (52.3%) and at the level of the aortic arch (43.1%) were the two main sites of origin, whereas the frequency of the site of origin above the azygos arch, at the level of the azygos arch, and below the azygos arch was 27.3%, 38.6%, and 34.1%, respectively. Considering both aortic and azygos arches, below the aortic arch and below the azygos arch were the most common sites of origin (27.3%). With respect to the running direction of TTB, in all cases, TTB passed below the azygos arch to the right upper lobe. There was no statistically significant (p > 0.05) difference in age or sex between types of TTB. Ancillary findings included tracheal stenosis (n = 2), narrowing of the right main bronchus (n = 2), luminal narrowing of TTB and bronchiectasis at the distal portion (n = 1), and a highly located azygos arch above the aortic arch (n = 2).
Conclusion
The proposed imaging classification of TTB and its anatomical relationship with surrounding structures will improve our understanding of various imaging features and embryological development of TTB. Radiologists should pay careful attention to evaluation of the airway including the trachea on thoracic imaging.
Tracheal bronchus is a congenital anomaly of bronchial division. A variety of bronchial anomalies originate from the trachea or main bronchus and are directed to the upper lobe territory (1). Tracheal bronchus usually originates from the main bronchus or trachea within 2 cm from the carina, and it supplies the entire upper lobe or apical segment of the upper lobe (234). The incidence of tracheal bronchus has been reported to range from 0.001% to 2% based on a bronchoscopy, autopsy, or radio-logical study. Tracheal bronchus may also be associated with other congenital anomalies such as Down's syndrome and congenital heart disease (5). Pig bronchus is an anomaly of the entire right upper lobe bronchus arising from the trachea. Tracheal bronchus can be divided into displaced type or supernumerary type, with the displaced type being more frequent than the supernumerary type. The supernumerary bronchi may end blindly. They are called tracheal diverticulum (12345678). In tracheal bronchus, an anomaly that originates only from the trachea is called true tracheal bronchus (TTB), which has a great clinical significance. It can be easily confirmed by surgery or bronchoscopy (2). Therefore in this study, we only focused on TTB.
Recently, CT findings of TTB have been studied using multi-detector computed tomography (MDCT) with multiplanar reconstruction and three-dimensional images (3589). Most articles on CT findings of TTB have focused on its incidence, classification, or accompanying anomalies. To the best of our knowledge, studies illustrating the anatomical relationship between TTB and the surrounding structures have been rarely reported (369). Therefore, the purpose of this study was to classify TTB radiologically using multiplanar reconstruction and three-dimensional images obtained by MDCT and to evaluate the anatomical relationship between TTB and the surrounding structures. Because it is difficult to perform precise differentiation of displaced type or supernumerary type at the lung periphery only by CT, we tried a new subspecialized classification of TTB according to the existence of right upper lobe bronchus originating from the right main bronchus, and the number of segmental bronchi originating from the trachea in this study.
This retrospective study was approved by the Institutional Review Board of our hospital. The requirement for obtaining informed patient consent was waived.
This study included 44 patients (2–80 years old, 24 males and 20 females; mean age of 51.3 years) who underwent MDCT examination between January 2010 and August 2015 at a single tertiary center in Korea.
All chest CTs were performed with a 64-channel multidetector scanner (LightSpeed VCT; GE Medical Systems, Milwaukee, WI, USA) and a 256-channel multidetector scanner (Brilliance iCT; Philips Medical Systems, Cleveland, OH, USA). CT images were restored as DICOM files after being reconstructed to a slice thickness of 1.25 mm and an interval of 1.25 mm. Using three-dimensional image analysis program (Portal workstation V2.6.0.32, Philips Medical Systems), we acquired coronal views, minimum intensity projection, and three-dimensional volume rendering (VR) images. CT images were reviewed by two radiologists (K.Y.T and K.H.J) with 26 and 3 years of experience in interpreting thoracic CT in consensus. We did not formally assess interobserver agreement.
In this article, TTB was defined as an anomalous bronchus arising from the trachea and directed to the right upper lobe. TTB was classified by analyzing axial, coronal and minimum intensity projection images, multi-planar reformation images, and VR images. We classified TTB into five types (Type I to Type V) according to the existence of right upper lobe bronchus originating from the right main bronchus and the number of branches of segmental bronchus originating from TTB (Fig. 1).
We evaluated the sites of origin and running directions of TTB based on its anatomical relationship with surrounding structures. The sites of origin of TTB were classified as follows: above the aortic arch, at the level of the aortic arch, below the aortic arch, above the azygos arch, at the level of azygos arch, and below the azygos arch. The associations between the types of TTB and gender, age, or the anatomical relationships were determined. We also reviewed ancillary findings such as changes in the diameter of the trachea and main bronchus.
The patient group for each type was too small to perform a statistical analysis of the anatomical relationship between each type and the surrounding structures. However, we were able to analyze the relationship with gender and age. We used Fisher exact test for gender and Kruskal-Wallis test for age. Statistical significance was attained when p value was less than 0.05. Type 5 was excluded from the statistical analysis because there was only one such case.
Based on the imaging classification of TTB, Type I occurred at a frequency of 47.7% (n = 21/44) (Fig. 2). Type II occurred at a frequency of 13.6% (n = 6/44) (Fig. 3). Type III occurred at a frequency of 11.4% (n = 5/44) (Fig. 4). Type IV occurred at a frequency of 25.0% (n = 11/44) (Table 1, Fig. 5). Type V occurred at a frequency of 2.0% (n = 1/44) (Fig. 6).
According to the site of origin of TTB, below the aortic arch (52.3%, n = 23/44) and at the level of the aortic arch (43.1%, n = 19/44) were the two main sites of origin. The frequency of the sites of origin above the azygos arch, at the level of the azygos arch, and below the azygos arch was 27.3% (n = 12/44), 38.6% (n = 17/44) and 34.1% (n = 15/44), respectively. In 10 out of the 11 cases of Type IV TTB, the site of origin was above the azygos arch (Fig. 5).
Considering both aortic and azygos arches, below the aortic arch and below the azygos arch were the most common sites of origin (27.3%). In Type I TTB, at the level of the aortic arch and at the level of the azygos arch were the most common sites of origin. Below the aortic arch and below the azygos arch were the most common sites of origin in Type III TTB (Fig. 4). At the level of the aortic arch and above the azygos arch were the most common sites of origin in Type IV TTB (Fig. 5).
With respect to different anatomical relationships between the running directions of TTB and the surrounding structures, in all cases, TTB passed below the azygos arch to the right upper lobe.
There was no statistically significant (p > 0.05) association between gender or age and the types of TTB. The diameter of the trachea was decreased in two cases (one case of Type II TTB and one case of Type III TTB) (Fig. 4). The diameter of the right main bronchus was lesser than that of the left main bronchus in two cases (one case of Type I TTB and one case of Type V TTB) (Fig. 6). One case showed luminal narrowing of the TTB and bronchiectasis at the distal portion. A highly located azygos arch above the aortic arch was observed in two cases (Fig. 2).
Tracheal bronchus, an anomaly arising from the right tracheal wall to upper lobe, was first described in 1785 (1). Although it is inappropriate anatomically, tracheal bronchus encompasses bronchial anomalies originating from the trachea or main bronchus (9). When any bronchus originates from the trachea, it is called TTB. In animals such as sheep, goats, camels, giraffes, and swans, the right upper lobe bronchus normally originates from the right trachea. The entire right upper lobe bronchus originating from the trachea is called pig bronchus. It is uncommon in humans with a frequency of 0.1% to 3%. Tracheal diverticuli vary in shape and location. They can be divided into congenital and acquired types. The supernumerary type of TTB may end blindly. It is called tracheal diverticulum in some studies. The diagnostic sensitivity of MDCT for TTB is 100% (348101112). Recognizing the presence of a tracheal anomaly is helpful while performing tracheal intubation and unilateral lung ventilation (1013). Also, most of these procedures are performed in the trachea rather than the main bronchus. In this study, we only focused on TTB since it is very significant clinically (2).
There are a few hypotheses about the pathogenesis of tracheal bronchus. Bremer (14) proposed that tracheal bronchus is caused by a failure of regression of tracheal buds in utero. He found an incidence of aberrant tracheal buds of 5% in 80 human embryos. This incidence is much higher than that in the general population. Most tracheal buds regress. Only a few tracheal buds will develop into tracheal bronchi or diverticuli. Another theory suggested that tracheal bronchus occurs as a result of disruption of normal embryogenesis (15). Alescio and Cassini (16) induced the development of tracheal buds by transplanting bronchial mesenchyme into tracheal epithelium.
In general, tracheal bronchus is subdivided into the displaced type or supernumerary type. An ectopic bronchus is supernumerary if the right upper lobe bronchus has normal trifurcation into apical, posterior, and anterior segmental bronchi. Tracheal bronchus is defined as the displaced type when the normal right upper lobar bronchus or segment is missing. In some studies, pig bronchus has been defined as a different type of TTB (51017). However, it is difficult to perform precise differentiation of the displaced type or supernumerary type at the lung periphery only by CT. Therefore, in this study, we tried another classification of TTB, according to the existence of right upper lobe bronchus originating from the right main bronchus and the number of segmental bronchi originating from the trachea. Our results revealed that Type I TTB was the most frequent (47.7%) type followed by Type IV TTB (25%). Suzuki et al. (3) reported that in 17 cases, a displaced TTB supplied all segments of the upper lobe (eight cases), apical segment (eight cases), and both apical and anterior segments (one case). TTB supplying the apical segment was classified as Type I TTB in this study. TTB supplying the apical and anterior segments was classified as Type II TTB. Pig bronchus, a TTB supplying all segments of the upper lobe, was classified as Type III. Suzuki et al. (3) reported the same frequency of Type I and III TTBs. However, in this study, Type I TTB was the most frequent. The frequencies of Type II and III TTBs were similar to each other. In this study, there was only one case of Type V TTB with one segmental bronchus originating from the trachea and absence of the right upper lobe bronchus originating from the right main bronchus. Since the right minor fissure was absent in this case, we suggested that it could be hypoplasia or lobar agenesis of the right upper lobe. To the best of our knowledge, such a case has not yet been reported.
In this study, the sites of origin of TTB were mostly below the aortic arch (52.3%) and at the level of the aortic arch (43.1%). Only two cases had sites of origin above the aortic arch. The sites of origin of TTB were not related to the location of the azygos arch. In two cases, the azygos arch was unusually highly located above the aortic arch (Fig. 2). Considering both aortic and azygos arches, below the aortic arch and below the azygos arch were the most common sites of origin.
In this study, the running direction of TTB was not related to the sites of origin. In every case, tracheal bronchus passed below the azygos arch to the right upper lobe. In utero, the trachea bifurcates at 4–6 weeks and branches progressively to form airways, while the azygos system develops generally at 6–7 weeks. Since the azygos vein starts to migrate from the lung apex after the development of tracheal bronchus, the downward migration of the azygos vein is blocked by TTB. Then, the azygos arch lies above the TTB. If TTB originates above the aortic arch, the azygos arch will also be located higher (3571819). In two cases included in this study, TTB passed below the highly located azygos arch (Fig. 2). The relationships between TTB and the surrounding structures have not yet been reported. This study provides useful information that will enable us to understand a variety of CT findings of TTB and offers a more subspecialized classification of TTB by using MDCT. This CT evaluation based on the anatomical relationship with the surrounding structures would result in increasing the success rate of surgery or intubation and decreasing the potential complications.
We could not obtain statistical information on the anatomical relationships between the types of TTB and the surrounding structures due to the small number cases of each type. Further study with more cases will be helpful to evaluate the anatomical relationship among types of TTB. Also, this more subspecialized classification could be helpful in clinical application.
There was no statistically significant association between gender or age and the types of TTB.
TTB is usually asymptomatic. Occasionally, it is the cause of relapsing cough and bronchitis as a result of retained secretion due to structural problems (3). In addition, there have been reports about bronchial stenosis, bronchiectasis, collapse, and accessory lobe related to TTB (310). Luminal narrowing of the TTB and bronchiectasis in the distal lung were found in one case included in this study, suggesting that they might have been caused by recurrent or chronic infection. Another case showed focal luminal narrowing of the trachea just proximal to the TTB. It might be associated with inflammation. In the study by Suzuki et al. (3), there was no case of tracheal stenosis. However, there were 10 cases of luminal narrowing of the TTB and there was one case of collapse with bronchiectasis of the right upper lobe. In two cases included in our study, the diameter of the right main bronchus was smaller than that of the left main bronchus (Fig. 6). Doolittle and Mair (5) reported one case of pig bronchus with luminal narrowing of the right main bronchus. They explained that right main bronchial stenosis might have occurred embryologically when the right upper lobe bronchus no longer contributes to the growth or the development of the main bronchus. In one case included in our study, distal tracheal stenosis after branching of TTB was found in a 49-year-old woman (Fig. 4) who had a history of recurrent bronchitis or chronic cough since childhood. Gower et al. (17) reported distal tracheal stenosis after branching of TTB in a 6-month-old boy who presented with cough and noisy breathing since 5 weeks of age. Distal tracheal stenosis after branching of TTB also suggests that the right upper lobe bronchus might have affected the growth of the trachea embryologically.
There were some limitations to our study. First, the classification used in this study was based on a new radiological approach. The existing classification divides TTB into the supernumerary and the displaced types. CT was unable to accurately detect the existence of normal bronchus in the peripheral potion of the lung by applying the classification proposed by Ghaye et al. (1). Second, there was a possibility of sample selection bias because we excluded patients who had only axial images. Third, although this study investigated more patients than previous studies, less than ten patients with different types of TTB led to difficulty in discussing the differences among types.
In conclusion, we tried to perform imaging classification of tracheal bronchus based on MDCT. In addition, we determined the anatomical relationships between TTB and the surrounding structures. This study will help improve our understanding of various imaging features and embryologic development of TTB. Also, the importance of evaluation of airway including the trachea should be emphasized in thoracic imaging.
Figures and Tables
Fig. 1
Imaging classification of true tracheal bronchus on MDCT.
Type I: Anomalous one segmental bronchus arising from the lower trachea to the right upper lobe with existence of the right upper lobe bronchus originating from the main bronchus. Type II: Anomalous two segmental bronchi arising from the lower trachea to the right upper lobe with existence of the right upper lobe bronchus originating from the main bronchus. Type III: The entire right upper lobe bronchus arising from the lower trachea without existence of the right upper lobe bronchus arising from the main bronchus. Type IV: Anomalous bronchus arising from the lower trachea with a blind end. Type V: Anomalous segmental bronchus arising from the lower trachea to the right upper lobe without existence of the right upper lobe bronchus arising from the main bronchus.
MDCT = multi-detector computed tomography
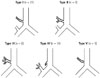
Fig. 2
True tracheal bronchus (Type I) in an asymptomatic 35-year-old man.
A. Axial CT image showing a highly located azygos arch (arrowhead) at the level of site of origin of thoracic great vessels.
B, C. Axial CT (B) and coronal minimum intensity projection (C) images showing true tracheal bronchus (open arrow) originating at the level of the azygos arch and running below the azygos arch.

Fig. 3
True tracheal bronchus (Type II) in an asymptomatic 37-year-old man.
Axial (A, B) and coronal minimum intensity projection (C) images showing anomalous two segmental bronchi (open arrow in A, C) arising from the lower trachea and one segmental bronchus (solid arrow in B, C) arising from the distal portion of the right main bronchus.

Fig. 4
True tracheal bronchus (Type III) in a 49-year-old man with chronic cough and dyspnea.
Coronal volume rendering (A) and minimum intensity projection (B) images showing an entire right upper lobe bronchus (open arrow) originating from the lower trachea. The lower trachea distal to the origin of tracheal bronchus is markedly narrowed. The diameter of the right main bronchus is less than or equal to that of the left main bronchus.
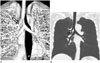
Fig. 5
True tracheal bronchus (Type IV) in an asymptomatic 53-year-old man.
Coronal volume rendering (A) and minimum intensity projection (MinIP, B) images showing an anomalous bronchus with a blind end (open arrow), arising from the lower trachea. It is also called tracheal diverticulum. On coronal MinIP (B) and serial axial CT (C) images, true tracheal bronchus ends blindly within the mediastinal fat, medial to the azygos arch (arrowhead).
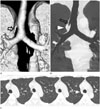
Fig. 6
True tracheal bronchus (Type V) in a 71-year-old man with chronic cough.
Coronal volume rendering (A) and minimum intensity projection (B) images showing an anomalous segmental bronchus (open arrow) arising from the lower trachea without existence of the right upper lobe bronchus arising from the right main bronchus. The diameter of the right main bronchus is less than that of the left main bronchus.
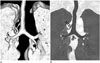
Table 1
Imaing Classification and Anatomic Assessment for TTB

References
1. Ghaye B, Szapiro D, Fanchamps JM, Dondelinger RF. Congenital bronchial abnormalities revisited. Radiographics. 2001; 21:105–119.
2. Ming Z, Lin Z. Evaluation of tracheal bronchus in Chinese children using multidetector CT. Pediatr Radiol. 2007; 37:1230–1234.
3. Suzuki M, Matsui O, Kawashima H, Takemura A, Matsubara K, Hayashi N, et al. Radioanatomical study of a true tracheal bronchus using multidetector computed tomography. Jpn J Radiol. 2010; 28:188–192.
4. Hong MJ, Kim YT, Jou SS, Park AY. Tracheobronchial branching anomalies. J Korean Soc Radiol. 2010; 63:149–159.
5. Doolittle AM, Mair EA. Tracheal bronchus: classification, endoscopic analysis, and airway management. Otolaryngol Head Neck Surg. 2002; 126:240–243.
6. Ulusoy M, Kivrak AS, Uysal II, Karabulut AK, Paksoy Y, Fazliogullari Z. Developmental anomalies of bronchial tree: a multidetector computerized tomography study. Int J Morphol. 2013; 31:1049–1055.
7. Barat M, Konrad HR. Tracheal bronchus. Am J Otolaryngol. 1987; 8:118–122.
8. Jou SS, Kim YT, Bae WK, Kim IY, Kim HH, Han JK. Evaluation of tracheal and main bronchial diverticula using thin-section MDCT. J Korean Soc Radiol. 2010; 62:123–130.
9. Beigelman C, Howarth NR, Chartrand-Lefebvre C, Grenier P. Congenital anomalies of tracheobronchial branching patterns: spiral CT aspects in adults. Eur Radiol. 1998; 8:79–85.
10. Gonlugur U, Efeoglu T, Kaptanoglu M, Akkurt I. Major anatomical variations of the tracheobronchial tree: bronchoscopic observation. Anat Sci Int. 2005; 80:111–115.
11. Miabi Z, Alaee A, Midia M, Hashemi H. Detection of rare congenital tracheal anomalies by multidetector CT in an infant. Acta Med Iran. 2006; 44:429–431.
12. Ritsema GH. Ectopic right bronchus: indication for bronchography. AJR Am J Roentgenol. 1983; 140:671–674.
13. Lee DK, Kim YM, Kim HZ, Lim SH. Right upper lobe tracheal bronchus: anesthetic challenge in one-lung ventilated patients -A report of three cases-. Korean J Anesthesiol. 2013; 64:448–450.
14. Bremer JL. Accessory bronchi in embryos; their occurrence and probable fate. Anat Rec. 1932; 54:361–374.
15. Reid L. 1976 Edward B.D. Neuhauser lecture: the lung: growth and remodeling in health and disease. AJR Am J Roentgenol. 1977; 129:777–788.
16. Alescio T, Cassini A. Induction in vitro of tracheal buds by pulmonary mesenchyme grafted on tracheal epithelium. J Exp Zool. 1962; 150:83–94.
17. Gower WA, McGrath-Morrow SA, MacDonald KD, Fishman EK. Tracheal bronchus in a 6-month-old infant identified by CT with three-dimensional airway reconstruction. Thorax. 2008; 63:93–94.
18. Buterbaugh JE, Erly WK. Paratracheal air cysts: a common finding on routine CT examinations of the cervical spine and neck that may mimic pneumomediastinum in patients with traumatic injuries. AJNR Am J Neuroradiol. 2008; 29:1218–1221.
19. Kosehan D, Kayıhan A, Koktener A. Incidental right paratracheal air cyst: significance of 64-detector multislice CT in differential diagnosis. New J Med. 2011; 28:62–63.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download