Abstract
Fibromatosis or desmoid tumor of the breast is a rare benign entity that has no metastatic potential but has significant risk of local recurrence. Its association with previous surgical or accidental trauma and Gardner's syndrome has been reported. Awareness of this lesion is important as the diagnosis is often confused with breast carcinoma. We report a case of a 44-year-old woman who presented with a palpable mass in her left breast, close to the axilla since a few months ago. She had undergone excisional biopsy for her left breast mass 15 months ago, and the diagnosis was confirmed as intraductal papilloma with atypical ductal hyperplasia. Subsequent ultrasound and core needle biopsy revealed stromal fibrosis. After 9 months, the mass showed an increase in its size and the anteroposterior to width ratio on ultrasonography compared to the previous examination, and final excisional biopsy confirmed the diagnosis of desmoid-type fibromatosis.
Fibromatosis or desmoid tumor of the breast is a rare benign entity, accounting for only 0.2% of primary breast tumors (1). It is characterized by an infiltrative and locally aggressive growth pattern with frequent recurrences, but it has no metastatic potential (23). It often mimics the clinical presentation and radiologic features of breast carcinoma, which makes it difficult to distinguish this entity from breast carcinoma. Here, we report a case of slow growing desmoid-type fibromatosis of the breast, more specifically, the axillary tail in terms of its location, in a patient with a previous history of surgical procedure on the ipsilateral breast.
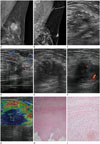
A 44-year-old woman presented with a palpable mass in her left breast close to the axilla since a few months ago. She had undergone excisional biopsy for her left breast mass 15 months ago, and the diagnosis was confirmed as intraductal papilloma with atypical ductal hyperplasia (Fig. 1A). On physical examination, the mass was about 2 cm in size, firm, nontender, and fixed. It was located in the 2 o'clock direction and 15 cm away from the nipple, close to the axilla. There was no accompanying skin abnormality. Mammogram showed asymmetry in the left axilla with a finding of postoperative state in the upper outer quadrant of the left breast (Fig. 1B). Subsequent ultrasonography revealed a 1.9 cm-sized, ill-defined irregularly shaped heterogeneously hypoechoic mass in the left axillary tail, at the palpable site. The adjacent subcutaneous layer showed increased echogenicity (Fig. 1C). The mass showed slightly increased vascularity at the periphery on color Doppler imaging (Fig. 1D). The lesion was classified as Breast Imaging Reporting and Data system (BI-RADs) category 4A, and a core needle biopsy under ultrasound guidance was performed. The histologic result revealed stromal fibrosis. After 9 months, follow-up ultrasonography was performed for the persistent palpable mass in the left axilla. The ultrasound examination demonstrated an ill-defined, irregular, heterogeneously hypoechoic mass in the left axillary tail. However, the size of the mass had increased to 2.5 × 1.7 × 2.4 cm, and the anteroposterior to width ratio had also increased compared to that in the previous examination (Fig. 1E). The mass showed vascularity at the periphery on color Doppler imaging (Fig. 1F). Ultrasound elastographic imaging showed a very low strain value in the hypoechoic mass, and an elasticity score of 4.5 was given according to the 5-point scoring system (Fig. 1G). The lesion was classified as BI-RADs category 4C, and subsequent core needle biopsy demonstrated stromal fibrosis with suspicion for fibromatosis. Therefore, excisional biopsy was performed. Histopathologic examination demonstrated an ill-defined nodular mass with abundant pinkish collagen fibers, and there was no identifiable breast parenchymal tissue in any field (Fig. 1H). The border of the mass was markedly irregular with streaks infiltrating into the adipose tissue. The surrounding skeletal muscle bundles were split by tumor cells. The cauterized resection margin was focally involved by tumor cells. Also, there were interlacing fascicles due to hypocellular spindle cell proliferation (Fig. 1I). The tumor cells were pin-pointed without marked cellular atypia or mitotic activity in the background of abundant collagen fibers. They were positive for smooth muscle actin and negative for desmin on immunohistochemical staining, compatible with myofibroblasts. Therefore, the diagnosis was confirmed as desmoid-type fibromatosis.
The latest follow-up mammogram performed at 1 year after excision demonstrated no evidence of local recurrence.
Fibromatosis or desmoid tumor of the breast is a slow-growing tumor that arises from the deep musculo-aponeurotic structures in a wide variety of anatomical locations (45). Desmoid-type fibromatosis of the breast is an exceedingly rare benign entity, accounting for only 0.2% of primary breast tumors. Although the tumor is benign and lacks metastatic potential, it is locally aggressive and may recur in up to 29% of cases (13). The etiology is not well understood, but its association with trauma and surgical procedure, such as silicone and saline breast implants, Gardner's syndrome, and familial multicentric fibromatosis has been reported (134). Although its occurrence is more common in young and fertile women, desmoid tumors have also been reported in men (13).
In this case, desmoid-type fibromatosis developed in a patient with a previous history of surgical procedure on the ipsilateral breast. Although aggressive fibromatosis is known to have an association with previous surgery, there are actually few case reports focusing on the patient group that had undergone a surgical procedure on the breast (4). What is more interesting in this case is the fact that desmoid-type fibromatosis arose some distance away from the previous scar. Previously excised and histologically confirmed intraductal papilloma was located in the 2 o'clock direction and in the middle-third on the mammogram, and a newly developed lesion was located in the 2 o'clock direction and 15 cm away from the nipple, close to the axilla. Furthermore, the lesion showed features of suspicious malignancy on serial imaging examinations with interval change in its appearance. Initially, we thought that it might be nonspecific inflammation in the accessory breast, but malignancy could not be excluded. After the follow-up examination, the mass was classified as BI-RADs category 4C, which led to excisional biopsy for pathologic confirmation.
Clinically, desmoid tumors of the breast present as firm, painless, and movable masses, and skin retraction and/or dimpling may be present. Nipple retraction is often seen in tumors that are close to the nipple, but nipple discharge and palpable lymphadenopathy are not associated with breast fibromatosis.
Mammographically, desmoid tumors are often irregularly shaped high density lesions with spiculated margins that closely mimic breast carcinoma (12). Rarely do these tumors demonstrate calcific deposition (3). On ultrasonography, breast fibromatosis typically presents as a solid spiculated or microlobulated irregular hypoechoic mass with poorly defined margins, findings that make it indistinguishable from breast cancer (2367). More benign appearances have also been reported and they are characterized by circumscribed borders and posterior acoustic enhancement (1). MRI is the best imaging technique for evaluating the tumor extent, and in particular, chest wall involvement (37). The masses are typically irregular, hypo- or isointense to muscle on T1-weighted images, and they show variable high signal intensity on T2-weighted images (138). They show suspicious slow enhancement after contrast administration (18).
It is characterized histologically by low-grade spindle cell proliferation comprising interlacing fibroblastic bundles and fascicles with varying degrees of collagen. The lesions are found to have irregular finger-like margins with spindle cells infiltrating and surrounding the normal breast parenchyma. Grossly, the lesion appears as a rubbery, poorly vascularized grayish-white mass. Although cellular atypia may be present, the spindle cells are typically uniform with a low mitotic index (24).
Management of desmoid-type fibromatosis of the breast includes wide local surgical excision because of high local recurrence rates, ranging from 24% to 77% over the course of 10 years (2). It may be difficult to clinically assess the margins during the surgical procedure because of the infiltrative nature of fibromatosis, and frozen sections may be helpful to determine clear margins (7). However, if the patient has had a previous biopsy, it may be difficult to differentiate between mammary fibromatosis and the prior biopsy site. Radiation therapy is used in patients with unresectable tumors or lesions that would require extensive surgical resection. Postoperative radiation therapy can improve the 10-year recurrence-free survival rate (1). Medical therapy includes three major classes of drugs: hormonal agents, anti-inflammatory agents, and cytotoxic agents (2).
In conclusion, breast fibromatosis may display suspicious malignant features on ultrasonography. But we can consider desmoid-type fibromatosis as one of the differential diagnoses for a mass mimicking breast carcinoma, especially in patients with a previous history of surgical procedure.
Figures and Tables
Fig. 1
Slow growing desmoid-type fibromatosis of the breast in a 44-year-old woman.
A. Initial left mammogram shows a 1.9 cm-sized oval shaped isodense mass with obscured margin in the upper outer quadrant, and the diagnosis was confirmed as intraductal papilloma with atypical ductal hyperplasia after excisional biopsy.
B. Follow-up left mammogram when the patient presented with a palpable mass in her left breast 15 months after excision shows asymmetry in the axilla (arrow).
C. Ultrasonogram performed at the same time as B shows an ill-defined, irregularly shaped, heterogeneously hypoechoic mass at the palpable site.
D. On color Doppler imaging, the mass shows slightly increased vascularity at the periphery.
E. Follow-up ultrasound imaging performed 9 months after C shows an ill-defined, irregular, and heterogeneously hypoechoic mass which has increased in its size and the anteroposterior to width ratio compared to that in previous examination.
F. The mass shows slightly increased vascularity at the periphery on color Doppler imaging.
G. Ultrasound elastographic imaging demonstrates a very low strain value in the hypoechoic mass, showing an elasticity score of 4.5 based on the 5-point scoring system.
H. Microscopic examination shows an ill-defined nodular mass with abundant pinkish collagen fibers and streaks infiltrating into the adjacent adipose tissue (hematoxylin and eosin stain, ×10).
I. Microscopic examination shows hypocellular spindle cell proliferation arranged in interlacing fascicles (hematoxylin and eosin stain, ×200).
References
1. Erguvan-Dogan B, Dempsey PJ, Ayyar G, Gilcrease MZ. Primary desmoid tumor (extraabdominal fibromatosis) of the breast. AJR Am J Roentgenol. 2005; 185:488–489.
2. Ha KY, Deleon P, Hamilton R. Breast fibromatosis mimicking breast carcinoma. Proc (Bayl Univ Med Cent). 2013; 26:22–24.
3. Glazebrook KN, Reynolds CA. Mammary fibromatosis. AJR Am J Roentgenol. 2009; 193:856–860.
4. Rosen PP, Ernsberger D. Mammary fibromatosis. A benign spindle-cell tumor with significant risk for local recurrence. Cancer. 1989; 63:1363–1369.
5. Otero S, Moskovic EC, Strauss DC, Benson C, Miah AB, Thway K, et al. Desmoid-type fibromatosis. Clin Radiol. 2015; 70:1038–1045.
6. Park JY, Ko MS, Kim HH, Shin HJ, Hwang JE, Gong GY, et al. Aggressive desmoid tumor mimicking breast cancer. J Korean Soc Radiol. 2010; 62:497–500.
7. Lim SJ, Kang YH, Kim L, Cho YU, Lee JW, Kim YJ. Recurrent primary fibromatosis in the breast: a case report. J Korean Soc Radiol. 2012; 66:287–291.
8. Nakazono T, Satoh T, Hamamoto T, Kudo S. Dynamic MRI of fibromatosis of the breast. AJR Am J Roentgenol. 2003; 181:1718–1719.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download