Abstract
Multiple intrahepatic arterial aneurysms and spontaneous aneurysmal rupture associated with polyarteritis nodosa leading to hemoperitoneum are extremely rare occurrences, but the conditions can be life-threatening if left untreated because of the risk of massive hemorrhage. We report a case of a high-risk surgical patient with polyarteritis nodosa complicated by spontaneous rupture of multiple intrahepatic arterial aneurysms. He was initially treated with emergency gelatin sponge particle embolization, followed by maintenance steroid treatment. Complete resolution of intrahepatic arterial aneurysms was observed at follow-up.
Polyarteritis nodosa (PAN) is an inflammatory, necrotizing vasculitis of the small- and medium-sized arteries (123). Hepatobiliary complications associated with PAN are unusual and intrahepatic hematoma and hemoperitoneum due to spontaneous rupture of a hepatic aneurysm have rarely been reported (345). We describe a case of spontaneous rupture of intrahepatic aneurysms in a patient initially managed with gelatin sponge particle embolization as an emergency treatment option, and immunosuppressants after the diagnosis of PAN was made. This report was approved by the Institutional Review Board of our hospital.
A 32-year-old man presented to the emergency department with seizures, sudden-onset epigastric pain, and mental deterioration. His medical history included congenital hydrocephalus, moderate mental retardation, hypertension, gait disturbance, and hepatitis B; he had no history of trauma. Physical examination revealed upper abdominal tenderness. He had a blood pressure of 80/60 mm Hg, pulse rate of 100 bpm, and body temperature of 35.4℃. Laboratory workup revealed reduced hemoglobin (5.8 g/dL) and hematocrit (17%) levels, and elevated aspartate transaminase (1697 units/L), alanine transaminase (350 units/L), alkaline phosphatase (497 units/L), total bilirubin (6.2 mg/dL), creatinine (1.7 mg/dL), and C-reactive protein (4.65 mg/dL) levels. Serological tests were positive for hepatitis B surface antigen, and negative for hepatitis C virus, human immunodeficiency virus, antinuclear antibodies, antineutrophil cytoplasmic antibodies, and rheumatoid factor.
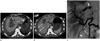
The abdominal computed tomography (CT) scan showed an intrahepatic hematoma measuring approximately 10 cm in diameter, extravasation of the contrast medium into the left medial segment, multifocal, small, contrast extravasations on the surface of the liver, and a subcapsular hematoma exceeding 3 cm in thickness, with a large amount of hemoperitoneum (Fig. 1A, B). The portal vein remained patent. There were no variations of the hepatic artery.
As the patient was not in an optimal condition to undergo surgery due to high likelihood of acute liver failure, emergency angiography was performed. A 5-French RH catheter [Cook (Cook Medical, Bloomington, IN, USA)] was advanced into the common hepatic artery via the femoral artery approach, and selective hepatic angiography was performed. The common hepatic angiogram showed multiple aneurysms of varying sizes, multifocal vascular ectasia, and stenoses of the intrahepatic arteries. In addition, extravasation of the contrast medium from the ruptured left medial hepatic artery was observed (Fig. 1C). Diverse collateral circulations were found at the distal portion of the bleeding focus. A 2.5-French superselective microcatheter [CANTATA (Cook Medical)] was inserted proximal to the left medial hepatic artery over a 0.016 inch microwire [Fathom (Boston Scientific, Naidich, MA, USA)], and embolization was performed using gelatin sponge particles (Cali-Gel 560–710 µm; Alicon, Hangzhou, China) mixed with contrast media. Following embolization and fluid volume resuscitation, the patient's vital signs slowly stabilized.
In view of diffuse muscular weakness, background of hypertension, elevated creatinine level (>1.5 mg/dL), the presence of hepatitis B surface antigen, and angiographic abnormalities, the patient was diagnosed with PAN, based on the American College of Rheumatology criteria (1990); conservative treatment with prednisolone and cyclophosphamide was initiated. After three weeks, his liver function tests stabilized and his general condition improved. He was discharged on prednisolone maintenance. At five weeks post-treatment, a follow-up angiogram showed almost complete resolution of multiple intrahepatic aneurysms, with no evidence of contrast extravasation. A non-enhanced abdominal CT performed 29 months later showed complete resolution of the intrahepatic hematoma and hemoperitoneum. Since the disease was in remission, the patient was able to begin steroid tapering.
PAN is a rare systemic vasculitis with an estimated incidence of 2–3 cases per million. Its incidence among hepatitis B patients is approximately 10 times higher than that in the general population (34).
Characteristic pathophysiology of PAN involves immune complex deposition in the vessel walls during the acute phase, allowing the formation of microaneurysms at the weakened points in the vessel walls (2367). Hepatitis B virus-induced immune response may be involved in the initial immune complex deposition (2). During progression to the chronic stage, thickening of the vessel walls occurs due to fibroblastic proliferation, which leads to arterial stenoses or occlusions, and causes ischemia or infarction in the affected organ (7). The most commonly involved organs are kidneys, but heart, gastrointestinal tract, liver, spleen, and pancreas may also be affected (347).
Clinical manifestations of PAN are very nonspecific, and they may include fever, polymyalgia, malaise, skin rash, arthralgia, weight loss, and abdominal pain. Furthermore, PAN has a pattern of exacerbation and remission, and there are no specific serological markers. Due to these reasons, the diagnosis and treatment of PAN may be delayed (3456).
Diagnosis of PAN can be confirmed by detection of focal necrotizing arteritis with polymorphonuclear neutrophil infiltration in biopsy of the affected organ (34). However, due to focal or segmental arterial involvement, biopsy has a sensitivity of only 70% (5), and depending on the clinical condition, the biopsy procedure itself may be high risk (6). Given the nonspecific nature of the clinical findings and laboratory test results of PAN, as well as the difficulties in the biopsy procedure, angiography plays an important role in the diagnostic confirmation of PAN (6). In this case, the patient had no definite clinical symptoms that could lead the physician to suspect PAN prior to admission, and his laboratory test results were nonspecific. However, the characteristic angiographic findings increased the likelihood of PAN as the diagnosis.
Characteristic angiographic findings of PAN include luminal irregularities resulted from ectasia and stenoses, multiple saccular or fusiform aneurysms, intra-arterial thromboses, and occlusive lesions in the small- and medium-sized arteries (368). Although CT can reveal hemorrhage sites or confirm the presence of organ infarction due to arterial occlusion, it is unable to detect the presence of a few small aneurysms, minimal luminal irregularities or narrowing (36). However, angiography in patients with PAN allows more specific detection of arterial abnormalities and visceral involvement such as organ infarction due to arterial occlusion or aneurysmal rupture (2). Furthermore, if required, concurrent embolization of the hemorrhage site is possible (28).
Hepatic arterial aneurysmal rupture secondary to PAN has been rarely reported. Hence, consensus on the guidelines for its management is limited. In general, hepatic arterial hemorrhage in a patient presenting with hypovolemic shock due to severe uncontrolled bleeding is managed with aggressive resuscitation and surgical treatment such as liver resection and hepatic artery ligation. In hemodynamically stable patients, transarterial embolization (TAE) should be the treatment of choice after confirmation of intrahepatic hemorrhage with an abdominal CT scan or angiogram (49). However, in cases of trauma, where venous bleeding may accompany arterial bleeding, surgical management is more appropriate (10). Because spontaneous aneurysmal rupture of the intrahepatic or perihepatic artery is a rare arterial complication of PAN, the possibility of venous bleeding can be safely excluded.
Non-surgical management of unruptured aneurysms due to PAN involves immunosuppressive therapy with steroids and cyclophosphamide, which leads to clinical improvement in most cases (4578). If a patient shows clinical healing, the aneurysms disappear or they are significantly decreased in size on the angiogram (8). Therefore, when clinical improvement is confirmed, there is no need to perform follow-up angiography for assessment of visceral arterial aneurysms (458). However, invisibility of aneurysms does not always indicate quiescence or remission of PAN (8). As we have already stated above, PAN typically shows a pattern of exacerbation and remission; hence, recurrence is always possible. When a vascular attack is suspected in a patient with PAN, diagnostic angiography should be performed immediately (8). Without treatment, the five-year survival rate is less than 15%; with immunosuppressive treatment, the survival rate increases up to 80% (37).
Parent et al. (4) reported cases in which laparotomy, arterial ligation, or coil embolization was used as the initial treatment option for intrahepatic aneurysmal rupture due to PAN. However, there are no case reports in which gelatin sponge particle embolization was used as the only treatment option.
Gelatin sponge particles are inexpensive, readily available, temporary occlusive agents. They can stop active bleeding immediately, but because the blockage is temporary and the artery recanalizes after two to three weeks, it appears that the artery is allowed to heal during this interval (9). In general, for cases with single arterial involvement, extensive arterial damage, or a precise embolization location, coil embolization is used (10). For cases with multiple arterial involvement, distal arterial damage, or presence of a diverse collateral circulation, a particulate agent like gelatin sponge particles or glue is utilized (10). If coil embolization is used for hepatic arterial aneurysms, it is essential to occlude both the distal and proximal sides of the site of injury to prevent retrograde flow from the collateral circulation to the embolized vessel (9). However, if gelatin sponge particles are used, there is no risk of retrograde flow; it is therefore possible to perform temporary complete arterial occlusion in shorter time.
As in the present case, emergency TAE using gelatin sponge particles is a possible treatment option for patients at high risk for surgery and with an angiogram showing hemorrhage in the distal portion of the intrahepatic artery with diverse collateral circulations. Additionally, with aggressive immunosuppressive therapy until recanalization, we can expect the unruptured aneurysms to decrease in size or resolve completely (358).
In summary, rupture of multiple hepatic arterial aneurysms is a rare but life-threatening complication of PAN. CT scan and angiography are important tools for diagnosing and making treatment decisions. In emergency situations where surgery may be difficult, gelatin sponge particle embolization is a safe and time-saving treatment option.
Figures and Tables
Fig. 1
Spontaneously ruptured intrahepatic arterial aneurysms in a 32-year-old man with polyarteritis nodosa who presented with a sudden-onset epigastric pain.
A. Axial contrast-enhanced CT shows an intrahepatic hematoma measuring approximately 10 cm in diameter in segment IV and extravasation of the contrast (arrow).
B. Multifocal small contrast extravasations (arrows) on the surface of the liver, and a subcapsular hematoma exceeding 3 cm in thickness with a large amount of hemoperitoneum are seen.
C. A selective common hepatic angiogram shows multiple aneurysms of varying sizes (arrows) with multifocal vascular ectasia and stenoses (arrowhead). Note the active extravasation of the contrast from the ruptured left medial hepatic artery (curved arrow), presumed to be extravasation of the contrast observed in segment IV on the CT scan.
References
1. Lightfoot RW Jr, Michel BA, Bloch DA, Hunder GG, Zvaifler NJ, McShane DJ, et al. The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum. 1990; 33:1088–1093.
2. Parangi S, Oz MC, Blume RS, Bixon R, Laffey KJ, Perzin KH, et al. Hepatobiliary complications of polyarteritis nodosa. Arch Surg. 1991; 126:909–912.
3. Stanson AW, Friese JL, Johnson CM, McKusick MA, Breen JF, Sabater EA, et al. Polyarteritis nodosa: spectrum of angiographic findings. Radiographics. 2001; 21:151–159.
4. Parent BA, Cho SW, Buck DG, Nalesnik MA, Gamblin TC. Spontaneous rupture of hepatic artery aneurysm associated with polyarteritis nodosa. Am Surg. 2010; 76:1416–1419.
5. Leung VK, Lam CY, Chan CC, Ng WL, Loke TK, Luk IS, et al. Spontaneous intra-hepatic haemorrhage in a patient with fever of unknown origin. Hong Kong Med J. 2007; 13:319–322.
6. Hagspiel KD, Angle JF, Spinosa DJ, Matsumoto AH. Diagnosis please. Case 13: polyarteritis nodosa--systemic necrotizing vasculitis with involvement of hepatic and superior mesenteric arteries. Radiology. 1999; 212:359–364.
7. Rhodes ES, Pekala JS, Gemery JM, Dickey KW. Case 129: polyarteritis nodosa. Radiology. 2008; 246:322–326.
8. Darras-Joly C, Lortholary O, Cohen P, Brauner M, Guillevin L. Regressing microaneurysms in 5 cases of hepatitis B virus related polyarteritis nodosa. J Rheumatol. 1995; 22:876–880.
9. Bauer JR, Ray CE. Transcatheter arterial embolization in the trauma patient: a review. Semin Intervent Radiol. 2004; 21:11–22.
10. Korean Society of Interventional Radiology. Interventional radiology. 2nd ed. Seoul: Ilchokak;2014. p. 368–370.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download