Abstract
Complete androgen insensitivity syndrome (AIS) is a rare, X-linked recessive disorder. Patients with AIS may develop primary amenorrhea due to androgen receptor resistance, resulting in a normal female phenotype and male (XY) karyotype. We report a case of a 30-year-old woman who was diagnosed with complete AIS. Ultrasonography and magnetic resonance imaging revealed bilateral inguinal cryptorchidism and no ovaries and uterus. After gonadectomy, the inguinal mass was confirmed as testicular atrophy with hamartomatous proliferation of Leydig cells and paratesticular leiomyoma. Although these tumors have been reported in association with AIS, this is the first case of paratesticular leiomyoma with hamartomatous proliferation of Leydig cells in atrophic testes being reported in Korea.
The complete form of androgen insensitivity syndrome (AIS) is a rare, X-linked disorder of hormone resistance characterized by a female phenotype, an XY karyotype, and the presence of testes that produce normal concentrations of androgens. The manifestations of complete AIS include primary amenorrhea, cryptorchidism, and infertility (12).
Radiologic imaging is useful to evaluate female genital organs and to localize cryptorchidism with gonadal tumors before surgery. Various tumors associated with the gonads have been confirmed by radiologic and pathologic findings. Among gonadal tumors that are associated with this syndrome, paratesticular leiomyoma has been reported in several cases worldwide (3). Herein, we report a patient with complete AIS and pathologically confirmed paratesticular leiomyoma who underwent ultrasonography (US) and magnetic resonance imaging (MRI). The study was approved by the Institutional Review Board.
A 30-year-old woman was referred for consultation and surgery after being diagnosed with complete AIS at a local gynecological clinic. The patient had been amenorrheic for her entire life, but had not sought any treatment. On physical examination, she had normal breast development and external genitalia, but her pubic and axillary hair were sparse. The patient had a small palpable mass in the right inguinal area. Serum testosterone and estradiol levels were 3.05 ng/mL and 39 pg/mL, respectively; both were within normal limits. Subsequent chromosomal analysis showed a 46, XY karyotype.
Transabdominal US revealed a heterogeneous hypoechoic oval mass with an abutting cystic component in both inguinal regions (Fig. 1A). Color Doppler imaging showed scant vascularity in the mass (Fig. 1B). On pelvic US, the uterus and ovaries were absent. To help localize the gonads, we performed pelvic MRI, which revealed the absence of the uterus and ovaries. The blind-ended vagina measured 5 cm in length (white arrow in Fig. 1C). MRI showed two solid masses, suggesting that both testes (hollow arrow in Fig. 1D, E) were located in the inguinal canals; the right mass, measuring 1.8 × 0.8 × 2.0 cm, was located in the superficial inguinal ring, while the left mass, measuring 1.8 × 1.2 × 2.6 cm, was located in the deep inguinal ring. Both testes had lower signal intensity than normal testes due to fibrosis and atrophic changes. An isointense T2 signal tubular structure close to the testes (white arrow in Fig. 1D, E) was observed, which appeared to be similar in shape to atrophic epididymides. Multiloculated cysts (asterisk in Fig. 1D-F) were visible adjacent to the lower pole of the testes. There were no signs of malignancy.
The patient subsequently underwent bilateral gonadectomy. Macroscopic examination revealed multiloculated cysts containing reddish fluid, in addition to solid gray and yellow areas. Histopathology of the mass revealed the presence of testes with atrophic seminiferous tubules and hamartomatous proliferation of Leydig cells (Fig. 1G). The tubular structure adjoining the mass, observed on MRI, contained smooth muscle fibers that stained positive for actin (Fig. 1H). This feature was consistent with leiomyoma. Leiomyoma perhaps originated from the paratesticular tissues (epididymis, spermatic cord, and vestigial remnants). Bilateral masses had the same pattern on microscopic examination. The multiloculated cystic wall was lined by stratified cuboidal epithelial cells. The final histopathologic diagnosis was testicular atrophy with hamartomatous proliferation of Leydig cells and paratesticular leiomyoma. Postoperatively, no complications occurred and the patient was scheduled to begin hormone replacement therapy.
AIS, previously known as testicular feminization, is an X-linked recessive disorder caused by mutations in the androgen receptor. This syndrome is relatively rare, with the estimated prevalence ranging from 1 in 20400 to 1 in 99100 genetic males. AIS is classified into complete and partial forms, depending on the presence and absence of virilization (1). Complete AIS is associated with the absence of functional androgen receptors, and the patients have male karyotypes with normal external female genitalia and secondary sexual characteristics. Furthermore, they have blind-ending vaginas, but lack uterus, ovaries, and fallopian tubes. In addition, they have cryptorchidism instead of female gonads. These manifestations are a result of defective binding of testosterone to the androgen receptor. Patients with partial AIS have some functional androgen receptors. Therefore, their phenotypic presentation includes micropenis, severe hypospadias, and a bifid scrotum (12). Our patient was diagnosed with complete AIS based on these characteristic manifestations.
Diagnosis of AIS is based mainly on clinical findings that present as symptoms such as amenorrhea, inguinal hernia, physical examination, and XY karyotype. Radiologic imaging may provide important information to the clinicians. US, computed tomography, and MRI can be used to reveal the absence of Müllerian duct derivatives and gonad location. Among these modalities, MRI is the imaging method of choice, with 100% accuracy for evaluation of Müllerian structures and gonad location (4). The position of the testes in a patient with AIS is highly variable. The gonads can be located anywhere in the abdomen, inguinal canal, or in the sublabial soft tissues (5), and they are commonly adjoined by cystic components that are thought to be remnants of Müllerian or Wolffian ducts (6). In our patient, we discovered incompletely descended testes with an adjoining cyst in bilateral inguinal rings, on both US and MRI.
The risk of malignancy in patients with complete AIS increases with age, from an estimated risk of 3.6% at 25 years to 33% by 50 years of age (7). Gonadectomy after puberty is recommended to avoid malignant transition within the testes. Our patient was 30 years of age at the time of diagnosis of complete AIS. Therefore, we performed prophylactic gonadectomy.
Various tumors have been described in association with cryptorchidism in AIS. The origin of the tumors can be from testicular stromal cells (Sertoli cell or Leydig cell tumors), testicular germ cells (seminoma), or other mesenchymal cells (lipoma, leiomyoma, and fibroma) (378). The current case had a paratesticular leiomyoma associated with cryptorchidism. Leiomyoma is extremely rare in gonads removed from patients with AIS, with a limited number of cases reported in the medical literature (59). Leiomyoma is the second most common neoplasm of the epididymis. On US, with the tumor abutting the testes, gross examination was unreliable in excluding malignancy. MRI findings suggested leiomyoma, which presented as a circumscribed lesion, with isointense signal on T1-weighted images, and hypointense regions on T2-weighted images compared with normal testes (10). To the best of our knowledge, no recurrence or malignant degeneration has ever been reported.
In conclusion, we presented a very rare case of complete AIS with paratesticular leiomyoma and hamartomatous proliferation of Leydig cells. It is important for patients with AIS to recognize the increased risk of developing malignancy after puberty. As seen in this case report, imaging is an important element in the initial diagnosis, preoperative planning, and treatment.
Figures and Tables
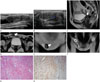 | Fig. 1Cryptorchidism and paratesticular leiomyoma with androgen insensitivity syndrome in a 30-year-old woman.
A. Transabdominal US reveals a heterogeneous hypoechoic oval mass with an abutting cystic component in both inguinal regions.
B. There is scant vascularity within the mass on Doppler US.
C. T2-weighted sagittal MR image showing a blind-ended vagina (white arrow) measuring 5 cm in length, without the uterus and ovaries.
D-F. T2-weighted axial (D) and coronal images (E, F) demonstrate a right testis (hollow arrow) in the superficial inguinal ring and a left testis in the deep inguinal ring. An isointense T2 signal tubular structure close to the testes (white arrow) is noted. Multiloculated cysts (asterisk) are visible adjacent to the lower pole of the testes.
G. Microscopic finding of the mass, showing the presence of testes with atrophic seminiferous tubules and hamartomatous proliferation of Leydig cells (hematoxylin and eosin statin, × 200).
H. Immunohistochemical staining reveals actin-positive smooth muscle fibers within the tubular structure (left side) adjoining the testes (right side) (× 200).
US = ultrasonography
|
References
1. Hughes IA, Davies JD, Bunch TI, Pasterski V, Mastroyannopoulou K, MacDougall J. Androgen insensitivity syndrome. Lancet. 2012; 380:1419–1428.
2. Khan S, Craig LB. A review of radiologic imaging in patients with androgen insensitivity. J Genit Syst Disord. 2013; S1. DOI: 10.4172/2325-9728.S1-003.
3. Siminas S, Kokai G, Kenny SE. Complete androgen insensitivity syndrome associated with bilateral Sertoli cell adenomas and paratesticular leiomyomas: case report and review of the literature. J Pediatr Urol. 2013; 9:e31–e34.
4. Sohaib SA, Cook G, Koh DM. Imaging studies for germ cell tumors. Hematol Oncol Clin North Am. 2011; 25:487–502, vii.
5. Nezzo M, De Visschere P, T'sjoen G, Weyers S, Villeirs G. Role of imaging in the diagnosis and management of complete androgen insensitivity syndrome in adults. Case Rep Radiol. 2013; 2013:158484.
6. Levin HS. Nonneoplastic diseases of the testis. In : Mills SE, Carter D, Greenson JK, Oberman HA, Reuter V, Stoler MH, editors. Sternberg's diagnostic surgical pathology. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins;2004. p. 2133–2166.
7. Iwamoto I, Yanazume S, Fujino T, Yoshioka T, Douchi T. Leydig cell tumor in an elderly patient with complete androgen insensitivity syndrome. Gynecol Oncol. 2005; 96:870–872.
8. Asl Zare M, Kalantari MR, Asadpour AA, Kamalati A. Bilateral laparoscopic gonadectomy in a patient with complete androgen insensitivity syndrome and bilateral sertoli-leydig cell tumor: a case report and brief review of the literature. Nephrourol Mon. 2014; 6:e15278.
9. Krichen Makni S, Mnif Hachicha L, Ellouze S, Mnif M, Khabir A, Ketata H, et al. [Feminizing testicular syndrome with multiple hamartomas and bilateral paratesticular leiomyomas]. Rev Med Interne. 2005; 26:980–983.
10. Tsili AC, Argyropoulou MI, Giannakis D, Sofikitis N, Tsampoulas K. MRI in the characterization and local staging of testicular neoplasms. AJR Am J Roentgenol. 2010; 194:682–689.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download