Abstract
Polyarteritis nodosa (PAN) is a systemic necrotizing vasculitis that involves medium- and small-sized arteries. PAN may affect any organ, and the presenting symptom of PAN varies depending on the organs affected. However, PAN generally spares the lung; thus, a report of PAN involving the bronchial artery is extremely rare, and hemoptysis has not been reported as the sole presenting symptom. Here, we report the case of a 39-year-old woman with hemoptysis who was diagnosed with PAN involving only the bronchial artery by angiography without involvement of the visceral arteries. Details of this case and a literature review are presented.
Polyarteritis nodosa (PAN) is a systemic necrotizing vasculitis that involves medium- and small-sized arteries (12). Patients with PAN typically present with systemic symptoms and specific signs related to the affected organs, most commonly the peripheral nerves, joints, skin, and kidney (1234). Thus far, there have been few reported premortem cases of bronchial artery involvement in PAN, but these cases had concomitant involvement of visceral arteries (125). In this report, we present a case of PAN involving only the bronchial artery, which presented as hemoptysis.
This research received a waiver of approval from the Dongguk University Ilsan Hospital Institutional Review Board (IRB). The requirement of obtaining informed consent was also waived by the IRB.
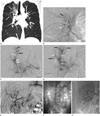
A 39-year-old woman was admitted to our hospital due to intermittent hemoptysis that developed 3 days before admission. One week earlier, she felt discomfort in her throat; 3 days before admission, she started coughing up blood intermittently and in small amounts. She had no specific medico-surgical history except for iron deficiency anemia. Her appearance was healthy, with no specific systemic symptoms such as fatigue, weight loss, or febrile sensation. Her vital signs were stable and her lung sounds were clear, without rales. Physical examinations related to her other systems did not yield remarkable results. Laboratory findings revealed a white blood cell count of 8640/µL, a hemoglobin level of 9.5 g/dL, a hematocrit of 30.3%, and a platelet count of 263,000/µL. The C-reactive protein (CRP) level was 0.03 mg/dL (range, 0–0.50 mg/dL) and the erythrocyte sedimentation rate (ESR) was 9 mm/hr (range, 0–20 mm/hr). Contrast-enhanced chest computed tomography (CT) showed ill-defined patchy diffuse ground glass opacities in the whole right lung, especially in the right upper and middle lobes (Fig. 1A), suggesting aspirated blood. There were no other abnormalities in the lung parenchyma, mediastinum, or pulmonary vessels on chest CT.
On the second hospital day, the patient underwent bronchoscopy, which revealed old blood clots in the trachea and in the branches of the right main bronchus but no specific endobronchial lesions. Immediately after the completion of bronchoscopy, the patient developed massive hemoptysis, and she started coughing up fresh blood. The estimated amount of hemoptysis was more than 300 mL immediately after bronchoscopy until the patient reached the angiography room.
She was intubated with a double-lumen endotracheal tube for one-lung ventilation and underwent emergency bronchial artery embolization (BAE). Angiograms of the right intercostobronchial trunk and common trunk of bilateral bronchial arteries showed a few small aneurysms in the proximal portion of the right bronchial artery. Additionally, multiple microaneurysms, supporting the diagnosis of PAN, were noted in peripheral branches of the right bronchial artery. Mild dilatation and tortuosity of the right bronchial artery were also seen (Fig. 1B-D). In the peripheral portion of the right bronchial artery, diffuse contrast staining similar to the tree-in-bud shape was noted. This finding was indicative of alveolar hemorrhage. Active extravasation of contrast medium into the right upper lobe bronchus was seen, together with diffuse filling defect lesions within the bronchus, suggesting hemo-bronchus. The right bronchial artery was successfully embolized using polyvinyl alcohol particles (150–250 µm, Bearing nsPVA; Merit Medical Systems, South Jordan, UT, USA), and no further active contrast extravasation was noted thereafter. Angiography of the visceral arteries was performed based on suspicion of systemic vasculitis with bronchial artery involvement. Angiography of the common hepatic artery, the superior mesenteric artery (SMA), and renal arteries did not show abnormal findings (Fig. 1E-G). Hemoptysis stopped immediately and the patient's condition was stable after BAE.
The angiographic findings of the bronchial artery suggested PAN. Additional laboratory tests were performed to exclude other possible diseases. Tests for auto-antibodies, including anti-nuclear antibody, rheumatoid factor, anti-glomerular basement membrane antibody, and anti-neutrophil cytoplasmic antibody (ANCA) against myeloperoxidase and proteinase 3, were negative. Infectious diseases were ruled out by blood and urine cultures and serologic studies. Based on the angiographic findings and other laboratory tests, she was diagnosed with PAN involving the bronchial arteries.
She was extubated 2 days after BAE and received high-dose glucocorticoid therapy along with intravenous cyclophosphamide. She is currently doing well, without any evidence or symptoms of systemic disease, after a follow-up period of 5 months.
PAN is a necrotizing vasculitis characterized by necrotizing inflammation of medium- and small-sized arteries that leads to necrosis and destruction of the vessel walls without glomerulonephritis or vasculitis of the arterioles, capillaries, and venules (16). PAN is a rare disease with an estimated annual incidence of 2.0–9.0 per million (1). PAN affects men more frequently than women, and the average age at disease onset is approximately 50 years (1). It may be idiopathic or secondary to a known cause (most likely hepatitis B virus infection), or it may manifest during the course of other rheumatologic diseases (1).
Typically, the clinical symptoms of PAN develop subacutely, with the onset of constitutional symptoms over weeks to months before the diagnosis of PAN is made (134). Laboratory findings include elevated CRP levels and an increased ESR (3). The patient described herein was unique as she had no constitutional symptoms, and both her ESR and CRP levels were normal. In addition, she presented only with hemoptysis due to involvement of only the bronchial artery. PAN is usually accompanied by specific signs associated with the organs affected, but this was not the case in our patient. The typical clinical symptoms are associated with occlusive changes in a vessel or aneurysmal rupture of an artery supplying the involved organ, including the musculoskeletal system, testis, gastrointestinal tract, liver, mesentery, skin, and heart, as well as non-glomerular renal vessels (134). PAN may affect any organ, but it generally spares the lung; thus, a report of PAN involving only the bronchial artery is extremely rare (1 5). Matsumoto et al. (7) reported bronchial arteritis in PAN in 7 of 10 postmortem cases. Lee et al. (5) reported the first premortem case of PAN involving the bronchial artery as well as both kidneys, the small bowel, and the liver in a patient who presented with hemoptysis and constitutional symptoms. This is the first report of PAN affecting only the bronchial artery, presenting as hemoptysis without involvement of visceral arteries such as the SMA, the hepatic artery, and renal arteries.
The diagnosis of PAN requires the integration of clinical, angiography, and biopsy findings (126). The key histopathologic finding of PAN is necrotizing inflammation of the medium- and small-sized arteries (126). In this patient, the involvement of the bronchial artery could not be confirmed by histopathology because of massive hemoptysis. However, the angiographic demonstration of characteristic changes suggests that the patient has arteritis involving medium-sized arteries, and accordingly, the diagnosis of PAN can be established even in the absence of histologic confirmation because direct pathologic demonstration in the involved arteries is not necessarily required to make a diagnosis (168). Typical angiographic findings of PAN are microaneurysms, aneurysms, ectasia, stenosis, and occlusion (12). The angiography findings in our patient, including aneurysms, microaneurysms, and irregular ectasia of bronchial arteries, were strongly suggestive of PAN. In this patient, the clinical symptoms were very different from those described in previous studies (18), such that the angiographic findings contributed decisively to the diagnosis. Additionally, systemic vasculitis, including ANCA-associated vasculitis [AAV; Wegener's granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome (CSS)] and PAN, should be classified and differentiated (9). Recently, a consensus algorithm for the classification of PAN and AAV has been developed and validated by combining the American College of Rheumatology criteria and Chapel Hill Consensus Conference definitions, ANCA testing and surrogate markers of vascular inflammation (9). This patient can be classified as having PAN according to this algorithm because the patient did not fulfill the criteria for CSS or Wegener's granulomatosis and was ANCA negative (9).
The prognosis of untreated PAN is very poor. The 1- and 5-year survival rates are 50% and 13%, respectively (35). The overall prognosis of this disease has improved during the past few decades primarily because of early diagnosis and treatment with systemic steroids, cyclophosphamide, and other immunosuppressive drugs, the current therapeutic agents of choice (1510). Relapse has been reported in up to 20% of idiopathic PAN cases (1).
In conclusion, although PAN involving the bronchial artery is extremely rare, the bronchial artery can be solely affected by PAN, in which case hemoptysis may be the initial and sole presenting symptom, unlike that in typical PAN patients. When systemic vasculitis is suspected based on the results of bronchial artery angiography and embolization, PAN should be included in the differential diagnosis. The appropriate tests should be performed to make a diagnosis, classify systemic vasculitis, and allow timely and optimal treatment.
Figures and Tables
Fig. 1
Isolated bronchial artery involvement by polyarteritis nodosa presenting as hemoptysis in a 39-year-old woman.
A. Initial coronal reformatted image of contrast-enhanced chest CT shows ill-defined patchy ground glass opacities in the right upper lung, particularly in the right upper lobe (arrows), suggesting aspirated blood.
B-D. Angiography was performed for treatment and diagnosis. Arteriogram of the right intercostobronchial trunk (B) shows an aneurysm in the proximal portion of the right bronchial artery (white arrow) and multiple microaneurysms in the peripheral portion of the right bronchial artery (black arrow). Arteriography of the common trunk of the right and left bronchial arteries (C) shows small aneurysms in the proximal portion of the right bronchial artery (white arrows), multiple small microaneurysms in the peripheral portion of the right bronchial artery (black arrows), and a filling defect lesion in the right upper lobe apical segment, suggestive of hemo-bronchus (empty arrow). Arteriography of the common trunk of the right and left bronchial arteries (D) shows diffuse alveolar hemorrhage in the right upper lobe (black arrows) and hemo-bronchus within the bronchi of the right upper lobe (empty arrows).
E-G. Angiographic findings of visceral arteries. Angiography of the common hepatic artery (E), angiography of the superior mesenteric artery (F), and angiography of the renal artery (G) show no abnormal findings.
CT = computed tomography
References
1. Colmegna I, Maldonado-Cocco JA. Polyarteritis nodosa revisited. Curr Rheumatol Rep. 2005; 7:288–296.
2. Lightfoot RW Jr, Michel BA, Bloch DA, Hunder GG, Zvaifler NJ, McShane DJ, et al. The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum. 1990; 33:1088–1093.
3. Pagnoux C, Seror R, Henegar C, Mahr A, Cohen P, Le Guern V, et al. Clinical features and outcomes in 348 patients with polyarteritis nodosa: a systematic retrospective study of patients diagnosed between 1963 and 2005 and entered into the French Vasculitis Study Group Database. Arthritis Rheum. 2010; 62:616–626.
4. Stone JH. Polyarteritis nodosa. JAMA. 2002; 288:1632–1639.
5. Lee YJ, Park SS, Kim SY, Lee JY, Koo HK, Yoon HI. A case of systemic polyarteritis nodosa involving bronchial artery. Sarcoidosis Vasc Diffuse Lung Dis. 2010; 27:164–168.
6. Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994; 37:187–192.
7. Matsumoto T, Homma S, Okada M, Kuwabara N, Kira S, Hoshi T, et al. The lung in polyarteritis nodosa: a pathologic study of 10 cases. Hum Pathol. 1993; 24:717–724.
8. Hernández-Rodríguez J, Alba MA, Prieto-González S, Cid MC. Diagnosis and classification of polyarteritis nodosa. J Autoimmun. 2014; 48-49:84–89.
9. Watts R, Lane S, Hanslik T, Hauser T, Hellmich B, Koldingsnes W, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis. 2007; 66:222–227.
10. Guillevin L, Cohen P, Mahr A, Arène JP, Mouthon L, Puéchal X, et al. Treatment of polyarteritis nodosa and microscopic polyangiitis with poor prognosis factors: a prospective trial comparing glucocorticoids and six or twelve cyclophosphamide pulses in sixty-five patients. Arthritis Rheum. 2003; 49:93–100.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download