Abstract
Purpose
To evaluate reproducibility of shear wave elastography (SWE) for breast lesions within and between observers and compare the reproducibility of SWE features.
Materials and Methods
For intraobserver reproducibility, 225 masses with 208 patients were included; and two consecutive SWE images were acquired by each observer. For interobserver reproducibility, SWE images of the same mass were ob-tained by another observer before surgery in 40 patients. Intraclass correlation coefficients (ICC) were used to determine intra- and interobserver reproducibility.
Results
Intraobserver reliability for mean elasticity (Emean) and maximum elasticity (Emax) were excellent (ICC = 0.803, 0.799). ICC for SWE ratio and minimum elasticity (Emin) were fair to good (ICC = 0.703, 0.539). Emean showed excellent ICC regardless of histopathologic type and tumor size. Emax, SWE ratio and Emin represented excellent or fair to good reproducibility based on histopathologic type and tumor size. In interobserver study, ICC for Emean, Emax and SWE ratio were excellent. Emean, Emax and SWE ratio represented excellent ICC irrespective of histopathologic type. ICC for Emean was excellent regardless of tumor size. SWE ratio and Emax showed fair to good interobserver reproducibility based on tumor size. Emin represented poor interobserver reliability.
Go to : 
Index terms
Breast, Ultrasonography, Elasticity Imaging Techniques, Breast NeoplasmREFERENCES
1. Tanter M, Bercoff J, Athanasiou A, Deffieux T, Gennisson JL, Montaldo G, et al. Quantitative assessment of breast lesion viscoelasticity: initial clinical results using supersonic shear imaging. Ultrasound Med Biol. 2008; 34:1373–1386.

2. Athanasiou A, Tardivon A, Tanter M, Sigal-Zafrani B, Bercoff J, Deffieux T, et al. Breast lesions: quantitative elastography with supersonic shear imaging–preliminary results. Radiology. 2010; 256:297–303.

3. Sewell CW. Pathology of benign and malignant breast dis-orders. Radiol Clin North Am. 1995; 33:1067–1080.
4. Hall TJ, Zhu Y, Spalding CS. In vivo real-time freehand pal-pation imaging. Ultrasound Med Biol. 2003; 29:427–435.

5. Konofagou EE, D'hooge J, Ophir J. Myocardial elastogra-phy–a feasibility study in vivo. Ultrasound Med Biol. 2002; 28:475–482.

6. Ophir J, Garra B, Kallel F, Konofagou E, Krouskop T, Righet-ti R, et al. Elastographic imaging. Ultrasound Med Biol. 2000; 26(Suppl 1):S23–S29.

7. Park CS, Kim SH, Jung NY, Choi JJ, Kang BJ, Jung HS. Interobserver variability of ultrasound elastography and the ultrasound BI-RADS lexicon of breast lesions. Breast Cancer. 2015; 22:153–160.

8. Chang JM, Moon WK, Cho N, Yi A, Koo HR, Han W, et al. Clinical application of shear wave elastography (SWE) in the diagnosis of benign and malignant breast diseases. Breast Cancer Res Treat. 2011; 129:89–97.

9. Cosgrove DO, Berg WA, Doré CJ, Skyba DM, Henry JP, Gay J, et al. Shear wave elastography for breast masses is highly reproducible. Eur Radiol. 2012; 22:1023–1032.

10. Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004; 51:396–409.

11. Bercoff J, Tanter M, Muller M, Fink M. The role of viscosity in the impulse diffraction field of elastic waves induced by the acoustic radiation force. IEEE Trans Ultrason Ferroelectr Freq Control. 2004; 51:1523–1536.

12. Bercoff J, Chaffai S, Tanter M, Sandrin L, Catheline S, Fink M, et al. In vivo breast tumor detection using transient elastography. Ultrasound Med Biol. 2003; 29:1387–1396.

13. Tozaki M, Fukuma E. Pattern classification of ShearWave™ Elastography images for differential diagnosis between benign and malignant solid breast masses. Acta Radiol. 2011; 52:1069–1075.
14. Yoon JH, Jung HK, Lee JT, Ko KH. Shearwave elastography in the diagnosis of solid breast masses: what leads to false-negative or false-positive results? Eur Radiol. 2013; 23:2432–2440.

15. Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006; 239:341–350.

16. Zhu QL, Jiang YX, Liu JB, Liu H, Sun Q, Dai Q, et al. Real-time ultrasound elastography: its potential role in assessment of breast lesions. Ultrasound Med Biol. 2008; 34:1232–1238.

17. Raza S, Odulate A, Ong EM, Chikarmane S, Harston CW. Us-ing real-time tissue elastography for breast lesion evaluation: our initial experience. J Ultrasound Med. 2010; 29:551–563.
18. Hudson JM, Milot L, Parry C, Williams R, Burns PN. Inter- and intra-operator reliability and repeatability of shear wave elastography in the liver: a study in healthy volunteers. Ultrasound Med Biol. 2013; 39:950–955.

19. Ferraioli G, Tinelli C, Zicchetti M, Above E, Poma G, Di Gre-gorio M, et al. Reproducibility of real-time shear wave elastography in the evaluation of liver elasticity. Eur J Radiol. 2012; 81:3102–3106.

20. Hiltawsky KM, Krüger M, Starke C, Heuser L, Ermert H, Jen-sen A. Freehand ultrasound elastography of breast lesions: clinical results. Ultrasound Med Biol. 2001; 27:1461–1469.

21. Yoon JH, Kim MH, Kim EK, Moon HJ, Kwak JY, Kim MJ. Interobserver variability of ultrasound elastography: how it affects the diagnosis of breast lesions. AJR Am J Roentgenol. 2011; 196:730–736.

22. Mun HS, Choi SH, Kook SH, Choi Y, Jeong WK, Kim Y. Vali-dation of intra- and interobserver reproducibility of shear-wave elastography: phantom study. Ultrasonics. 2013; 53:1039–1043.

23. Ng WL, Rahmat K, Fadzli F, Rozalli FI, Mohd-Shah MN, Chandran PA, et al. Shearwave elastography increases diagnostic accuracy in characterization of breast lesions. Medicine (Baltimore). 2016; 95:e3146.

24. Kim SJ, Ko KH, Jung HK, Kim H. Shear wave elastography: is it a valuable additive method to conventional ultrasound for the diagnosis of small (≤2 cm) breast cancer? Medicine (Baltimore). 2015; 94:e1540.
Go to : 
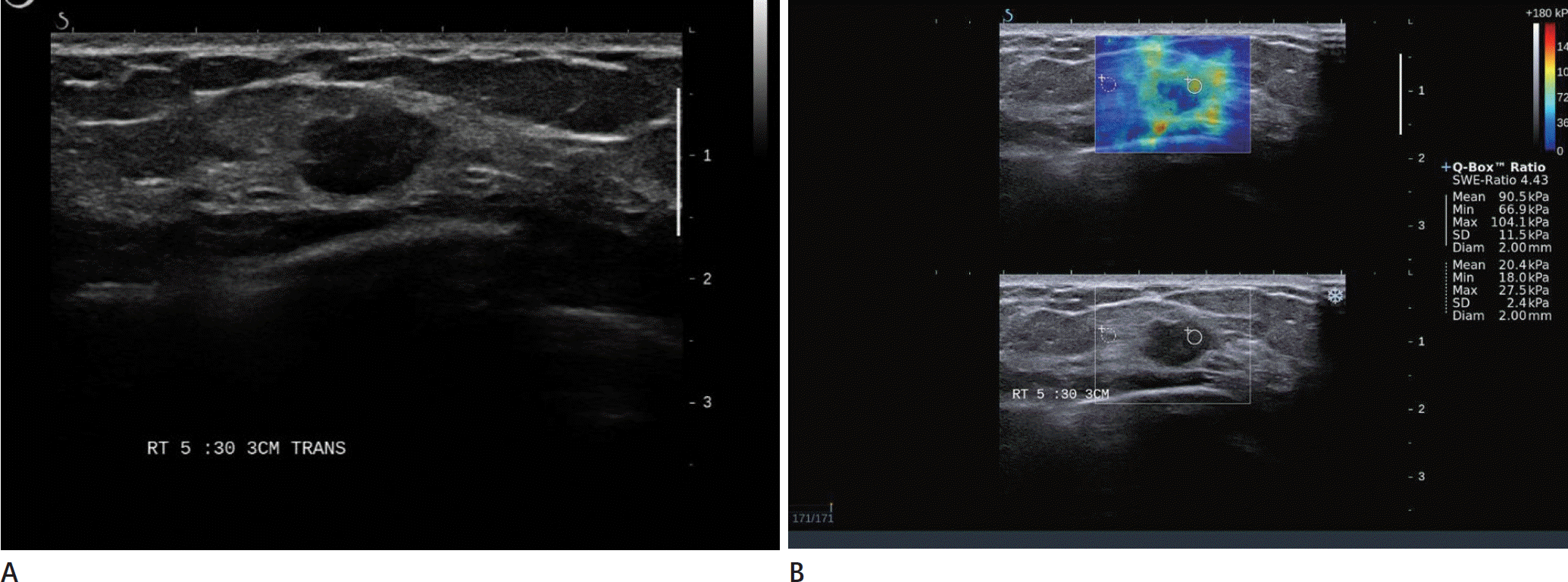 | Fig. 1.Conventional US and elastographic images of invasive ductal carcinoma in a 49-year-old woman. A. Conventional US image shows an irregular hypoechoic lesion without circumscribed margin. B. Shear wave elastography images of quantification box (Q-BoxTM) applies on the stiffest part of mass. An additional Q-BoxTM is placed in the ad-jacent surrounding tissue. US = ultrasonography |
Table 1.
Pathologic Diagnosis of 228 Breast Lesions in 208 Patients
Table 2.
Intraobserver Reproducibility of Quantitative SWE Measurements
| Overall | Benign | Malignancy | ||||
|---|---|---|---|---|---|---|
| ICC | 95% CI | ICC | 95% CI | ICC | 95% CI | |
| SWE ratio∗ | 0.703 | 0.606 to 0.801 | 0.636 | 0.452 to 0.821 | 0.668 | 0.555 to 0.780 |
| Emean† | 0.803 | 0.760 to 0.845 | 0.754 | 0.571 to 0.876 | 0.762 | 0.692 to 0.833 |
| Emax‡ | 0.799 | 0.744 to 0.855 | 0.744 | 0.648 to 0.889 | 0.769 | 0.667 to 0.822 |
| Emin§ | 0.539 | 0.419 to 0.660 | 0.491 | 0.115 to 0.868 | 0.498 | 0.360 to 0.637 |
Table 3.
Intraobserver Reproducibility of Quantitative Shear Wave Elastography Measurements in Patients with Invasive Cancer Versus with Ductal Carcinoma in situ
| Ductal Carcinoma in situ | Invasive Cancer | |||
|---|---|---|---|---|
| ICC | 95% CI | ICC | 95% CI | |
| SWE ratio∗ | 0.832 | 0.688 to 0.976 | 0.650 | 0.531 to 0.770 |
| Emean† | 0.879 | 0.788 to 0.970 | 0.744 | 0.681 to 0.806 |
| Emax‡ | 0.897 | 0.821 to 0.973 | 0.718 | 0.637 to 0.799 |
| Emin§ | 0.677 | 0.394 to 0.959 | 0.475 | 0.319 to 0.631 |
Table 4.
Intraobserver Reproducibility of Quantitative SWE Measurements according to the Mass Diameter
| Diameter ≤ 10 mm | 10 mm < Diameter ≤ 15 mm | 15 mm < Diameter ≤ 22 mm | Diameter > 22 mm | |||||
|---|---|---|---|---|---|---|---|---|
| ICC | 95% CI | ICC | 95% CI | ICC | 95% CI | ICC | 95% CI | |
| SWE ratio∗ | 0.718 | 0.585 to 0.852 | 0.634 | 0.412 to 0.856 | 0.746 | 0.622 to 0.870 | 0.644 | 0.446 to 0.842 |
| Emean† | 0.869 | 0.811 to 0.927 | 0.758 | 0.626 to 0.831 | 0.760 | 0.680 to 0.839 | 0.804 | 0.732 to 0.875 |
| Emax‡ | 0.891 | 0.848 to 0.934 | 0.752 | 0.665 to 0.839 | 0.740 | 0.656 to 0.825 | 0.770 | 0.646 to 0.893 |
| Emin§ | 0.592 | 0.412 to 0.772 | 0.603 | 0.443 to 0.762 | 0.504 | 0.301 to 0.707 | 0.435 | 0.157 to 0.712 |
Table 5.
Interobserver Reproducibility of Quantitative SWE Measurements
| Overall | Benign | Malignancy | ||||
|---|---|---|---|---|---|---|
| ICC | 95% CI | ICC | 95% CI | ICC | 95% CI | |
| SWE ratio∗ | 0.787 | 0.714 to 1.000 | 0.902 | 0.535 to 1.000 | 0.752 | 0.687 to 1.000 |
| Emean† | 0.909 | 0.558 to 1.000 | 0.879 | 0.486 to 1.000 | 0.906 | 0.481 to 1.000 |
| Emax‡ | 0.868 | 0.661 to 1.000 | 0.951 | 0.596 to 1.000 | 0.831 | 0.588 to 1.000 |
| Emin§ | 0.394 | 0.000 to 0.828 | 0.745 | 0.183 to 1.000 | 0.341 | 0.000 to 0.797 |
Table 6.
Interobserver Reproducibility of Quantitative SWE Measurements according to the Mass Diameter
| Diameter ≤ 15 mm | Diameter > 15 mm | |||
|---|---|---|---|---|
| ICC | 95% CI | ICC | 95% CI | |
| SWE ratio∗ | 0.423 | 0.565 to 1.000 | 0.760 | 0.587 to 1.000 |
| Emean† | 0.878 | 0.000 to 0.856 | 0.903 | 0.343 to 1.000 |
| Emax‡ | 0.653 | 0.169 to 1.000 | 0.888 | 0.560 to 1.000 |
| Emin§ | 0.217 | 0.000 to 0.743 | 0.278 | 0.000 to 0.766 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download