Abstract
Purpose
The purpose of this study was to evaluate the independent predictive factors for local tumor progression (LTP) of colorectal liver metastasis (CRLM) after radiofrequency ablation (RFA).
Materials and Methods
Patients with CRLM were included in the analysis if nod-ules were up to five in number, each nodule was ≤ 5 cm, and RFA was performed in our center from January 2006 to December 2015. Univariate and multivariate anal-yses to identify the predictors of LTP were performed by using a Cox proportional hazard model.
Results
Overall, 58 tumors from 38 patients were included in this study. LTP oc-curred in 14 tumors from 9 patients. The overall 1- and 3-year LTP rates were 23.5% and 29.4%, respectively. Multivariate analysis showed that tumor size > 2 cm and insufficient ablative margin were two independently significant adverse prognostic factors for LTP (p = 0.045 and 0.022, respectively). The 3-year LTP rates for 33 and 25 tumors with and without sufficient ablative margin were 4.5% and 61.2%, respectively. The difference was statistically significant (p < 0.001). The difference in the 3-year LTP rates according to the tumor size was not statistically significant (p = 0.791).
Go to : 
Index terms
Local Neoplasm Recurrence, Neoplasm Metastasis, Radiofrequency Thermal Ablation, Colorectal Neoplasm, LaparoscopyREFERENCES
1. Mulier S, Ruers T, Jamart J, Michel L, Marchal G, Ni Y. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? An update. Dig Surg. 2008; 25:445–460.
2. Wu YZ, Li B, Wang T, Wang SJ, Zhou YM. Radiofrequency ablation vs hepatic resection for solitary colorectal liver metastasis: a meta-analysis. World J Gastroenterol. 2011; 17:4143–4148.
3. Bismuth H, Adam R, Lévi F, Farabos C, Waechter F, Casta-ing D, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996; 224:509–520. discussion 520-522.

4. Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, et al. Anatomical major resection versus nonanatomical lim-ited resection for liver metastases from colorectal carcinoma. Am J Surg. 2001; 181:153–159.

5. Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995; 19:59–71.

6. Berber E, Tsinberg M, Tellioglu G, Simpfendorfer CH, Siperstein AE. Resection versus laparoscopic radiofrequency thermal ablation of solitary colorectal liver metastasis. J Gastrointest Surg. 2008; 12:1967–1972.

7. Hur H, Ko YT, Min BS, Kim KS, Choi JS, Sohn SK, et al. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg. 2009; 197:728–736.

8. Lee WS, Yun SH, Chun HK, Lee WY, Kim SJ, Choi SH, et al. Clinical outcomes of hepatic resection and radiofrequency ablation in patients with solitary colorectal liver metastasis. J Clin Gastroenterol. 2008; 42:945–949.

9. Veltri A, Sacchetto P, Tosetti I, Pagano E, Fava C, Gandini G. Radiofrequency ablation of colorectal liver metastases: small size favorably predicts technique effectiveness and survival. Cardiovasc Intervent Radiol. 2008; 31:948–956.

10. Aloia TA, Vauthey JN, Loyer EM, Ribero D, Pawlik TM, Wei SH, et al. Solitary colorectal liver metastasis: resection de-termines outcome. Arch Surg. 2006; 141:460–466. discussion 466-467.
11. Livraghi T, Solbiati L, Meloni F, Ierace T, Goldberg SN, Ga-zelle GS. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the “test-of-time approach”. Cancer. 2003; 97:3027–3035.
12. Lee KH, Kim HO, Yoo CH, Son BH, Park YL, Cho YK, et al. Comparison of radiofrequency ablation and resection for hepatic metastasis from colorectal cancer. Korean J Gastroenterol. 2012; 59:218–223.

13. Abitabile P, Hartl U, Lange J, Maurer CA. Radiofrequency ablation permits an effective treatment for colorectal liver metastasis. Eur J Surg Oncol. 2007; 33:67–71.

14. Berber E, Siperstein A. Local recurrence after laparoscopic radiofrequency ablation of liver tumors: an analysis of 1032 tumors. Ann Surg Oncol. 2008; 15:2757–2764.

15. Minami Y, Kudo M. Radiofrequency ablation of liver metastases from colorectal cancer: a literature review. Gut Liver. 2013; 7:1–6.

16. Machi J, Uchida S, Sumida K, Limm WM, Hundahl SA, Oishi AJ, et al. Ultrasound-guided radiofrequency thermal ablation of liver tumors: percutaneous, laparoscopic, and open surgical approaches. J Gastrointest Surg. 2001; 5:477–489.

17. Lang EV, Chen F, Fick LJ, Berbaum KS. Determinants of in-travenous conscious sedation for arteriography. J Vasc Interv Radiol. 1998; 9:407–412.

18. Shady W, Petre EN, Gonen M, Erinjeri JP, Brown KT, Covey AM, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes–A 10-year experience at a single center. Radiology. 2016; 278:601–611.

19. Funai T, Osugi H, Higashino M, Kinoshita H. Estimation of lymph node metastasis by size in patients with intratho-racic oesophageal cancer. Br J Surg. 2000; 87:1234–1239.

20. Alvarez S, Añorbe E, Alcorta P, López F, Alonso I, Cortés J. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol. 2006; 186:1342–1348.

21. Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: stan-dardization of terminology and reporting criteria–a 10-year update. J Vasc Interv Radiol. 2014; 25:1691–1705.e4.

22. Tanis E, Nordlinger B, Mauer M, Sorbye H, van Coevorden F, Gruenberger T, et al. Local recurrence rates after radiofrequency ablation or resection of colorectal liver metastases. Analysis of the European Organisation for Research and Treatment of Cancer #40004 and #40983. Eur J Cancer. 2014; 50:912–919.

23. Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The mini-mal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010; 195:758–765.
Go to : 
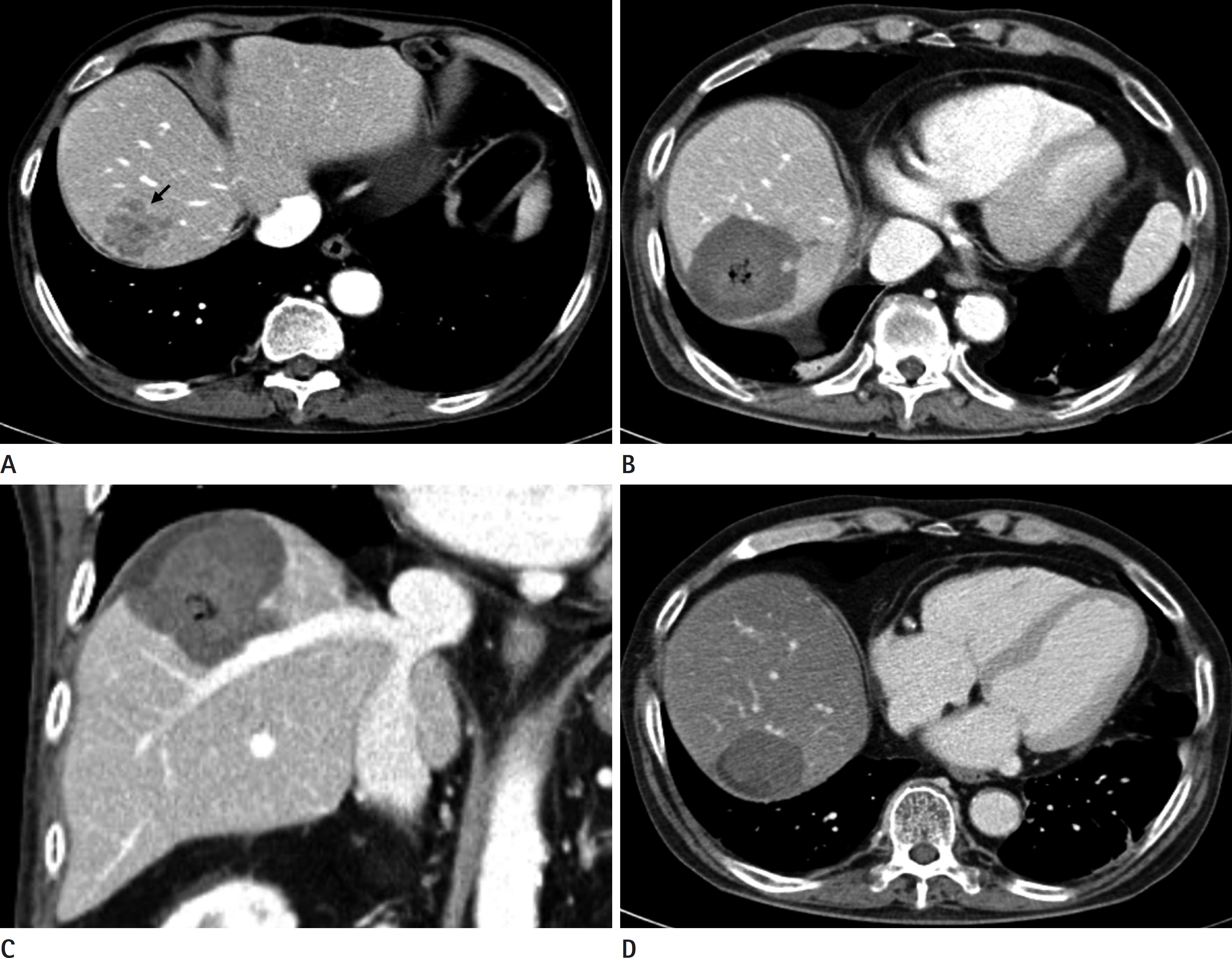 | Fig. 1.A 68-year-old male patient with rectosigmoid colon cancer. A. A 3.6 cm sized hypodense nodule with peripheral rim enhancement (black arrow) is newly detected in the right lobe of the liver on initial abdomen CT, suggesting liver metastasis. B, C. Eight days after intraoperative RFA using multiple separable electrodes, followup axial (B) and coronal (C) CT show a 5.8 cm hypodense ablation zone with a sufficient ablative margin surrounding the ablation index tumor. D. Nine months after intraoperative RFA, the ablation zone is markedly decreased in size without evidence of local recurrence. RFA = radiofrequency ablation |
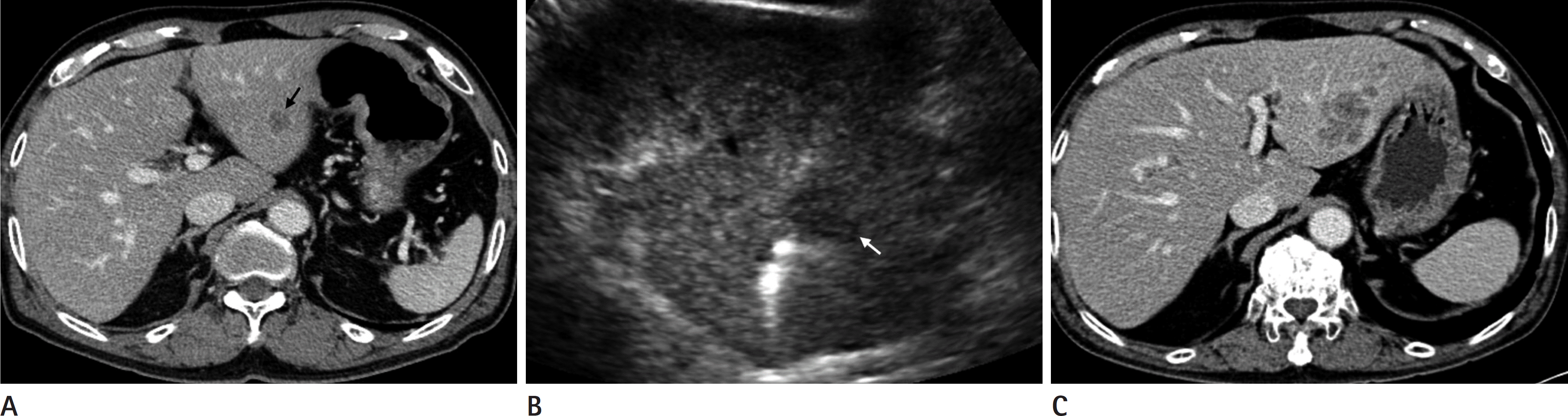 | Fig. 2.A 77-year-old male patient with rectal cancer. A. A 1.9 cm sized, hypodense nodule with peripheral rim enhancement (black arrow) is newly detected in the left lobe of the liver on followup abdomen CT, suggesting liver metastasis. B. Percutaneous RFA was conducted using an internally cooled single electrode. White arrow indicates the ablation index tumor. C. A CT image taken five months after RFA shows that the previous ablation zone is markedly increased in extent, and multiple liver metastases are newly developed in the other sites. RFA = radiofrequency ablation |
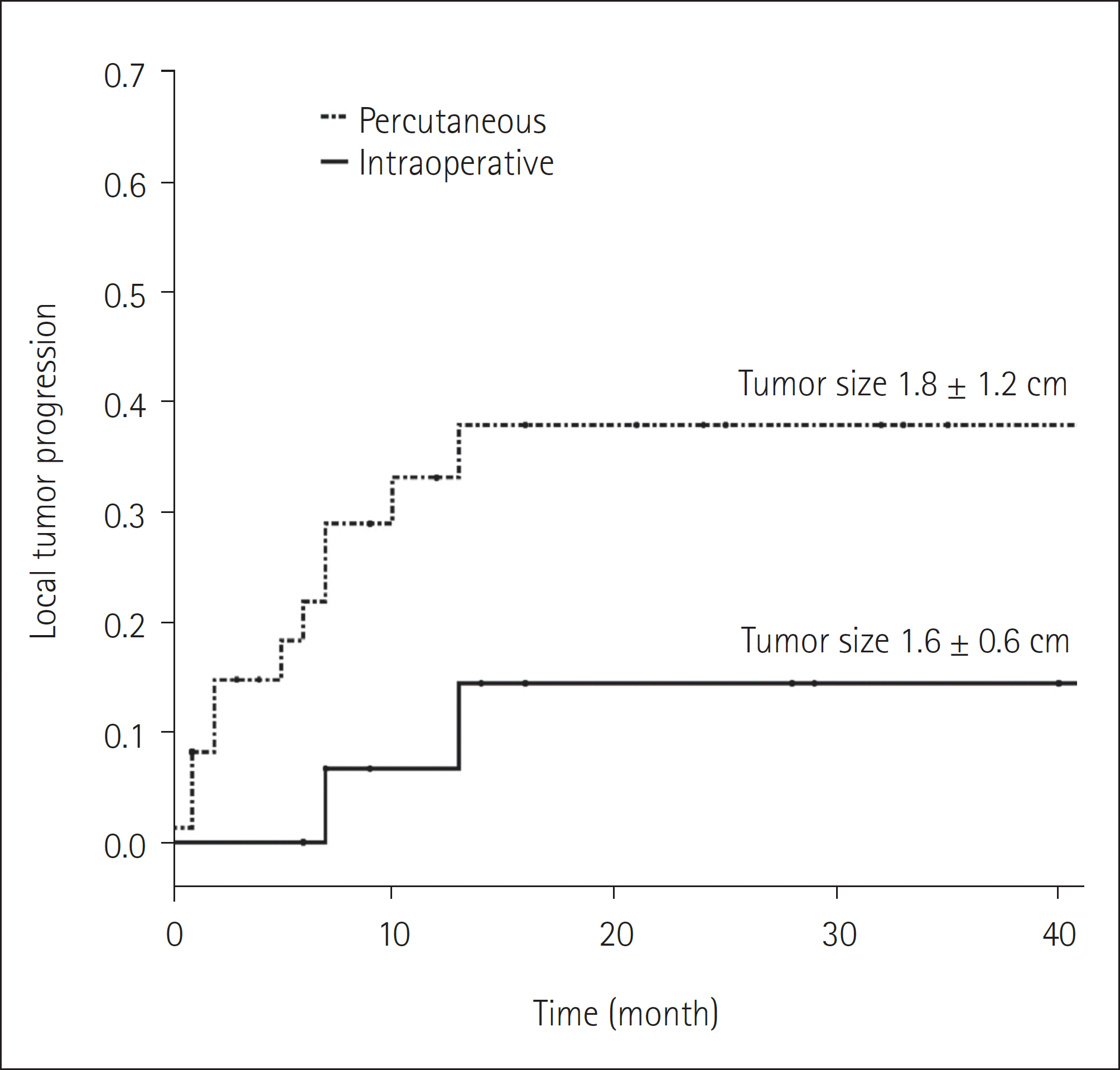 | Fig. 3.A comparison of the LTP rates for colorectal liver metastasis between the patients, confined to the subgroup, undergoing percutaneous and intraoperative RFA. The 3-year LTP rates after RFA using the intraoperative or percutaneous approach are significantly different; 14.4% vs. 37.9%, with statistical significance (p = 0.033). LTP = local tumor progression, RFA = radiofrequency ablation |
Table 1.
A Comparison of the Baseline Characteristics Per Nodule
Table 2.
Univariate Analysis of Prognostic Factors for the Local Tumor Progression of Colorectal Liver Metastasis after RF Ablation Per Nodule
| Hazard Ratio | Standard Error | p-Value | |
|---|---|---|---|
| Age ≥ 70 years old | 0.584 (0.161–2.111) | 0.656 | 0.412 |
| Male gender | 24.111 (0.013–43445.559) | 3.825 | 0.405 |
| Tumor size > 2 cm | 1.169 (0.364–3.756) | 0.596 | 0.793 |
| Multiple tumors | 1.785 (0.613–5.202) | 0.546 | 0.288 |
| Adjacent to large intrahepatic vessels > 3 mm in the diameter | 0.399 (0.052–3.077) | 1.042 | 0.378 |
| Presence of a dome nodule | 0.572 (0.128–2.559) | 0.764 | 0.465 |
| Presence of a subcapsular tumor | 0.607 (0.212–1.740) | 0.537 | 0.353 |
| Adjuvant chemotherapy | 0.942 (0.321–2.769) | 0.550 | 0.914 |
| Synchronous lung metastasis | 1.474 (0.461–4.710) | 0.593 | 0.513 |
| Synchronous lymph node invasion | 0.048 (0.000–149409.647) | 7.632 | 0.690 |
| Serum level of CEA >10 ng/mL | 2.693 (0.901–8.052) | 0.559 | 0.076 |
| Presence of other malignancies | 2.319 (0.301–17.880) | 1.042 | 0.420 |
| Intraoperative approach | 0.228 (0.051–1.023) | 0.765 | 0.054 |
| RF electrode type | 0.235 | ||
| Internally cooled single | 1 (reference group) | − | − |
| Internally cooled clustered | 0.261 (0.055-1.240) | 0.795 | 0.091 |
| Others | 0.678 (0.202-2.274) | 0.618 | 0.529 |
| Ablative margin ≥ 0.5 cm | 0.102 (0.023-0.458) | 0.767 | 0.003∗ |
Table 3.
Multivariate Analysis of Prognostic Factors for the Local Tumor Progression of Colorectal Liver Metastasis after RF Ablation Per Nodule
| Hazard Ratio | Standard Error | p-Value | |
|---|---|---|---|
| Tumor size > 2 cm | 10.363 (1.405–76.44) | 1.020 | 0.022∗ |
| Serum level of CEA >10 ng/mL | 2.260 (0.681–7.502) | 0.612 | 0.183 |
| Intraoperative approach | 0.435 (0.049–3.864) | 1.114 | 0.455 |
| RF electrode type | 0.304 | ||
| Internally cooled single | 1 (reference group) | − | − |
| Internally cooled clustered | 0.129 (0.012–1.404) | 1.217 | 0.093 |
| Others | 0.592 (0.160–2.191) | 0.667 | 0.433 |
| Ablative margin ≥ 0.5 cm | 0.105 (0.011–0.955) | 1.128 | 0.045∗ |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download