Abstract
Fibrous hamartoma of infancy (FHI) is a rare, benign subcutaneous tumor occurring mainly before the age of 2 years. The most commonly reported locations of FHI are the extremities such as the shoulder or axilla. However, FHI arising in the genital area is extremely rare and has not been reported with correlated radiologic findings. In this case report, we present a case of 5-month-old male child diagnosed with FHI in the scrotum, with a focus on the correlation between the radiologic and pathologic findings.
Fibrous hamartoma of infancy (FHI) is a rare, benign subcutaneous tumor which is solitary and painless, and it occurs before the age of 2 years (1). Pathologically, FHI is characterized by the presence of fibrous tissue, spindle cells in a mucoid matrix, and mature fat. The most common locations of FHI are the shoulder, axilla and upper arm, although it can be detected in any other part of the body (2). The occurrence of FHI in the genital area has been sporadically reported (3). However, to the best of our knowledge, there have been no reports of radiological findings of FHI in the scrotum. FHI has the potential to be confused with malignant tumors because of its rapid growth rate. Therefore, a preoperative diagnosis of FHI with clinical and radiological findings can be helpful in preventing overtreatment. We present a rare case of FHI in the scrotum, with a focus on the correlation between the radiologic and pathologic findings.
A previously healthy 5-month-old male infant presented to the outpatient clinic of our hospital's Urology Department with a painless, palpable mass in the right inguinal area which had been detected three months ago. Upon physical examination, the mass was found to be firm, fixed and attached to the skin of the scrotum. There was no evidence of inflammation such as heat sensation or redness of the skin. There were no significant abnormalities at birth or afterwards according to the patient's medical history. Routine laboratory findings were within the normal range. After a three-month follow-up, the mass showed rapid growth and continued to be hard or firm and fixed.
Sonography was performed in the patient utilizing an Aloka ProSound Alpha 7 ultrasound system (Hitachi Avius, Tokyo, Japan). Sonography showed a fusiform shaped, ill-defined, multi-layered mass, measuring approximately 1.0 × 0.4 × 0.8 cm in dimensions, in the right scrotum. The mass showed a mainly homogeneous hyperechogenicity compared to the surrounding subcutaneous fat and the testis. There was no calcification, necrosis or invasion into the testis or epididymis (Fig. 1A). There was an oval shaped hypoechoic testis without any connection with or invasion by the mass, inferior to the mass mentioned above (Fig. 1B). On Doppler image, the lesion revealed scanty internal vascularity but it was surrounded by feeding vessels (Fig. 1C).
Multi-detector computed tomography images were obtained with a 32 channel dual source CT scanner (SOMATOM Definition, Siemens Medical Solution. Forchheim, Germany). On the CT scan, an approximately 1.5 × 1.7 × 2.0 cm sized solid mass was located in the skin and subcutaneous layer of the right scrotum. In the pre-contrast enhanced phase, the mass showed low attenuation compared to the testis and it contained a fat component that had a spongiform appearance and mottled fat attenuation (mean HU: −59 HU) (Fig. 2A). On the post-contrast enhanced scan, it showed heterogeneous enhancement with the feeding vessels arising from the inferomedial portion of the mass (Fig. 2B). On axial scan of the post contrast enhanced CT (Fig. 2C) and upon sagittal reconstruction, the spermatic cord was slightly displaced posterior to the mass and it did not show a direct connection with the mass. This indicated that the tumor did not originate from the spermatic cord (Fig. 2D).
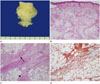
The mass was excised. The resected specimen was composed of skin, subcutaneous fat and soft tissue. Upon cross-sectional analysis, the cut surface was white tan and homogenous (Fig. 3A). Histologic examination showed that the extent of the mass was limited below the dermis (Fig. 3B). In the high magnification view, the mass was poorly circumscribed and it was made up of 3 components; 1) stellate immature mesenchymal cells in a myxoid matrix, 2) fibrocollagenous tissue composed of bland fibroblasts, and 3) mature fat (Fig. 3C). After performing immunohistochemical staining, we obtained a positive reaction for CD34 (Fig. 3D) and a negative reaction for S100 protein in the mesenchymal cells, confirming the presence of immature mesenchymal cells. A positive reaction for S100 protein in the fat component confirmed the presence of fat cells. On histologic examination, the mass was diagnosed as FHI.
FHI is an uncommon tumor, accounting for less than 2% of soft tissue tumors occurring in the first year of life (2). These tumors are rare, benign, subcutaneous soft tissue tumors appearing shortly after birth or at birth. FHI is more common in boys and the median age at presentation is ten months (4). It is reported that most FHI tumors occur before the age of 2 years (1).
FHI usually appears as a single tumor in the axilla, shoulder, digit, extremity, and trunk. Nevertheless, these tumors may also develop at other unusual sites such as the groin and scrotum (5). If a palpable mass is detected in the genital area, the differential diagnosis should include benign lesions such as incarcerated hernia, epidermal cyst, inclusion body fibromatosis, and infantile fibromatosis. FHI, especially in the genital region is frequently reported to be misdiagnosed as lymphadenopathy, rhabdomyosarcoma or malignancies arising from the neural tissue (4). It is very rare, but the occurrence of FHI in the genital area has been sporadically reported. In 1994, Popek et al. (3) described the pathological findings of 15 cases of genital FHI, which involved the inguinal region in five cases, the scrotum in five cases, the spermatic cord in one case, the perineum in one case, the labium majors in one case, the suprapubic region in one case, and the pubic area in one case.
FHI is considered to be a benign tumor despite its rapid infiltrative growth rate and local recurrence. It has the potential to be confused with malignant tumors because of its rapid growth rate and pathologic findings such as high degree of cellularity (6). Furthermore, a focal malformation that resembles a neoplasm in the tissue of its origin with poor margin and fibrous tendrils can also be detected. Thus, it is very important to differentiate it from other soft tissue masses.
Superficial soft-tissue masses are often diagnosed clinically. However, imaging studies are useful for determination of differential diagnoses, multiplanar localization, tissue characterization and proper size measurement.
Sonography shows a hyperechogenic, multilayered lobular mass with intratumoral large vessels and multiple septae (7). MRI is usually recommended for assessing pediatric soft tissue tumors because of excellent tissue characterization. It reflects the components of FHI. The fat components of FHI are hyperintense on T1-weighted images and they show reduced signal intensity on T1-weighted images with fat suppression. The fibrous tissues are usually hypointense on T1 and T2 weighted images (8). CT manifestations of FHI include fat density and dot-like calcification (9).
Pathologic characteristics of FHI were first described by Reye (10) in 1956, and they described the tumor as “subdermal fibromatous tumor in infancy”. Enzinger, in his review of 30 cases of FHI, described the common histopathologic feature of the tumor being composed of three types of tissue; connective, adipose, and mesenchymal. The criteria for histologic diagnosis include the presence of well-defined transversing bundles of dense, uniform, fibrous connective tissue, nodular aggregation of immature components lacking significant atypia, pleomorphism, and growth in a truly aggressive manner (28).
Sonographic evaluation of our case showed a heterogeneous hyperechoic mass with multilayering surrounded by feeding vessels. These sonographic findings corresponded to FHI, as mentioned in previous studies. But because of the ill-defined margin caused by similar echogenicity of the soft tissue surrounding the mass, we considered the possibility of malignancy. The pathologic report described the mass as poorly circumscribed and organized with adipose tissue and fibrocollagenous tissue which showed similar echogenicity to the surrounding soft tissue. We suggested that the ill-defined margin of the mass was caused by these pathologic characteristics of FHI. In our case, CT findings were useful in the differential diagnosis of the mass that did not involve the surrounding structures. In the CT image, the mass only compressed the other structures such as the spermatic cord. In the sagittal reconstruction images, the intact fat plane between the mass and the spermatic cord was well-detected.
Distinctive pathologic features of FHI have some correlation with imaging findings, as observed in our case. The multilayering and serpentine pattern of the tumor indicates that the tumor is not only composed of a single type of tissue. According to the pathologic reviews, the connective, adipose and mesenchymal components of FHI caused multilayering (7). Three components of FHI show different echogenicities and densities on imaging evaluation with sonography and computed tomography. Fibrous tissue is represented by the intervening hypoechoic portion in the hyperechoic mass on sonography and by hyperdensity on computed tomography. Adipose tissue is represented as the surrounding hyperechoic portion or internal multiple hyhypodense foci of the mass.
It is sometimes difficult for clinicians to diagnose FHI because it is rare and unfamiliar with them. Imaging evaluation of FHI can provide useful information such as the presence of fat and fibrous components within the lesion, location of the tumor, and the relationship with its surrounding structures. These findings can be helpful in the diagnosis and treatment of FHI.
Figures and Tables
Fig. 1
A 5-month-old boy with fibrous hamartoma of infancy; sonographic evaluation of the tumor in the right scrotum.
A. Transverse scan shows a relatively ill-defined, elliptical shaped, enlarged, multi-layered, heterogeneously hyperechogenic solid mass (arrow) compared to the surrounding subcutaneous fat, measuring approximately 1.0 × 0.4 × 0.8 cm in dimensions, which does not contain calcification or necrosis.
B. Inferior to the mass, the right testis (arrow) is found without any connection with the mass. The mass shows hyperechogenicity compared to the testis.
C. On Doppler image, the lesion reveals scanty internal vascularity but it is surrounded by feeding vessels.
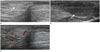
Fig. 2
A 5-month-old boy with fibrous hamartoma of infancy; contrast enhanced CT evaluation of the abdomen.
A. On axial scan, pre contrast enhanced abdominal CT shows a well-defined mass with internal mottled fat components (arrows) with fat attenuation (-59 HU) and it shows low attenuation compared to the testis.
B. On axial scan, post contrast enhanced abdominal CT shows a heterogeneously enhancing mass. Feeding vessels are present in the inferomedial portion of the mass (arrow).
C. On axial scan, post contrast enhanced abdominal CT shows that the spermatic cord (arrowhead) is slightly displaced posterior to the mass and it does not show a direct connection with the mass.
D. Sagittal reconstruction of post contrast enhanced abdominal CT also shows that the spermatic cord (arrowhead) is displaced posteriorly.
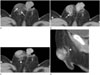
Fig. 3
A 5-month-old boy with fibrous hamartoma of infancy; histopathologic evaluation of the tumor.
A. On the cross-sectional view of the tumor, the cut surface is white tan and homogenous.
B. A photomicrograph shows the extent of the mass below the dermis (below the dotted line) (hematoxylin & eosin, × 12.5).
C. High magnification view of the photomicrograph shows mature adipose tissue (arrowhead), spindle shaped fibroblastic cells in the collagenous stroma (arrow) and immature round mesenchymal cells (hematoxylin & eosin, × 100).
D. Immunohistochemical staining shows a positive CD34 reaction in the mesenchymal cells, which indicates the presence of immature mesenchymal cells (CD34 stain, × 100).
References
1. Jeong IH, Kim JH, Kim SH. Fibrous hamartoma of infancy (FHI) developed in trunk. J Korean Neurosurg Soc. 2004; 36:331–333.
2. Enzinger FM. Fibrous hamartoma of infancy. Cancer. 1965; 18:241–248.
3. Popek EJ, Montgomery EA, Fourcroy JL. Fibrous hamartoma of infancy in the genital region: findings in 15 cases. J Urol. 1994; 152:990–993.
4. Sengar M, Mohta A, Manchanda V, Khurana N. Paratesticular fibrous hamartoma in an infant. Singapore Med J. 2012; 53:e63–e65.
5. Ferro F, Caterino S, Boldrini R, Bosmann C, Cavallini M. Fibrous hamartoma of infancy in the scrotum. Pediatr Surg Int. 1988; 4:71–73.
6. Stock JA, Niku SD, Packer MG, Krous H, Kaplan GW. Fibrous hamartoma of infancy: a report of two cases in the genital region. Urology. 1995; 45:130–131.
7. Rho BH, Lee HJ, Kwon SY. Imaging findings of fibrous hamartoma of infancy. J Korean Soc Radiol. 2009; 61:189–192.
8. Ashwood N, Witt JD, Hall-Craggs MA. Fibrous hamartoma of infancy at the wrist and the use of MRI in preoperative planning. Pediatr Radiol. 2001; 31:450–452.
9. Guo YK, Ning G, Zhao FM, Qu HB. Fibrous hamartoma of infancy mimicking teratoma in the parapharyngeal space on multidetector row CT. Pediatr Radiol. 2011; 41:785–787.
10. Reye RD. A consideration of certain subdermal fibromatous tumours of infancy. J Pathol Bacteriol. 1956; 72:149–154.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download