Abstract
Mammary-type myofibroblastoma is a very rare, benign mesenchymal tumor consisting of spindle-shaped cells along with thick hyalinized collagen bundles and an intralesional fat component; its histopathological features are identical to those of myofibroblastomas of the breast. It usually occurs along the embryonic milk-line; however, unusual cases occurring outside of the embryonic milk-line have also been reported. Although this tumor always shows clinically benign behavior, its variable histological composition can easily be confused with many other fibrous and lipomatous neoplasms. Unfortunately, its radiological findings are extremely rarely described in the literature. Here, we present a rare case of mammary-type myofibroblastoma in a 38-year-old woman who presented with a well-circumscribed solitary mass in the buttock, and discuss various radiologic imaging findings, such as plain radiography, ultrasonography, and magnetic resonance imaging results.
Mammary-type myofibroblastoma is an uncommon mesenchymal tumor that is histologically identical to myofibroblastoma of the breast, which was first described in 1987 by Wargotz et al. (1). Mammary-type myofibroblastoma, also called extramammary myofibroblastoma, occurs in sites outside the breast. It has been established that mammary-type myofibroblastomas commonly originate from the embryonic milk-line, extending from the mid-axilla to the medial groin (2). Although mammary-type myofibroblastomas are commonly reported in the iliac area, they are rarely reported in the axillae, mid-back, retroperitoneum, or buttocks (23). In fact, to the best of our knowledge, only two previous cases of mammary-type myofibroblastoma involving the buttocks have been reported. However, the radiologic findings of ultrasonography (USG) and magnetic resonance imaging (MRI) were not described in these previous reports. Thus, we here present a rare case of mammary-type myofibroblastoma occurring in the buttock and describe its USG and MRI findings.
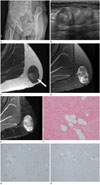
A 38-year-old woman visited our hospital for evaluation of a palpable mass located in the buttock that had an insidious onset. On physical examination, the palpable mass was found to be a well-circumscribed, firm and solitary mass measuring 7 × 5 cm, and it was asymptomatic on palpation. She had no history of trauma to the area and no evidence of premature ovarian failure. She also denied any previous history of excessive alcohol consumption or medication intake, including oral contraceptives, and she had no significant family history. The preliminary laboratory tests were unremarkable. For further evaluation, radiological imaging studies were performed. Oblique plain pelvic radiographs showed a well-defined, oval soft mass in the left buttock, without mineralization within the mass (Fig. 1A), whereas USG revealed a well-defined soft mass with a lobulated contour, measuring 6 cm, in the subcutaneous layer of the buttock (Fig. 1B). It also showed an echogenic lesion with relatively heterogeneous echotexture and variable posterior acoustic shadowing. Interestingly, there was some internal vascularity within the mass on color Doppler imaging. On subsequent MRI, a soft mass with internal low signal intensity was observed on T2-weighted imaging of the left buttock (Fig. 1C, D) and heterogeneous components within the tumor were also detected. T1-weighted imaging without fat suppression revealed high signal intensity in the central area of the mass and suppressed signal in the fat saturation sequence, suggesting the presence of an intratumoral fat component. The dense fibrous tissue of the tumor was visualized as low signal intensity on both T1- and T2-weighted images. Some vascular structures were detected in the peripheral aspect of the tumor (Fig. 1E).
Due to these radiological findings, the patient underwent wide excision, as the possibility of malignant potential could not be excluded. Macroscopically, the tumor was a well-circumscribed, firm and white-to-gray mass measuring 6 × 5 × 4 cm. Microscopically, the tumor was composed of slender fibroblast-like spindle cells along with extensive thick collagen bundles and trapped mature adipose tissue (Fig. 1F). The spindle cells had inconspicuous nucleoli and many cells were frequently noted; however, mitosis and necrosis were not evident. Spindle cell lipoma, cellular angiofibroma, and fibromatosis were considered as the main differential diagnoses. However, the trapped adipose tissue accounted for less than 10% of the tumor and the blood vessels were inconspicuous in the tumor. Most spindle tumor cells were positive for CD34 (Fig. 1G), desmin (Fig. 1H), estrogen receptor, and CD10, whereas they were negative for smooth muscle actin, S-100, and b-catenin. Taken as a whole, the tumor findings were consistent with mammary-type myofibroblastoma located in an extra-mammary site. The patient has been monitored for 18 months after wide excision and there has been no disease recurrence to date.
Mammary-type myofibroblastoma is a benign mesenchymal neoplasm that is histologically identical to myofibroblastoma of the breast, but it occurs in extra-mammary sites (12). Previous studies have shown that the most common site of mammary-type myofibroblastoma is the inguinal or groin area along the embryonic milk-line (34). Mammary-type myofibroblastomas are rarely found in sites such as the axilla, retroperitoneum, mid-back, or buttock (2). To date, only three case reports have described the radiographic findings of these tumors, including MRI findings (567). We herein report the first case of mammary-type myofibroblastoma located in the buttock, with descriptions of the USG and MRI findings.
Mammary-type myofibroblastomas are generally well-circumscribed, and they comprise spindle cells admixed with thick hyalinized collagen bands and interrupted mature adipocytes (8). The spindle tumor cells show immunoreactivity for desmin, vimentin, and CD34, whereas they do not show any reactivity ag-ainst S100 protein, epithelial membrane antigen, cytokeratins, or c-Kit (CD117) (910).
Unfortunately, mammary-type myofibroblastoma shows overlapping morphological and immunohistochemical features with spindle cell lipoma and cellular angiofibroma. However, unlike mammary-type myofibroblastoma, spindle cell lipomas do not express desmin, and collagen deposition varies slightly; these tumors show “rope-like” collagen fibers or diffuse sheets of collagen or myxoid matrix. On the other hand, cellular angiofibromas stain negative for desmin and comprise uniform, short spindle cells with an edematous component consisting of stromal delicate collagen fibers and numerous small-to-medium-sized hyalinized blood vessels. In cases of diagnostic dilemmas, the distinction among these tumors is somewhat arbitrary. In the current case, the spindle cells showed positive staining for both desmin and CD34, and the cells were admixed with thick collagen bundles. The morphological and immunohistochemical findings in our case are similar to those of myofibroblastoma of the breast. In addition, the mature adipose tissue component occupied less than 10% of the tumor and variable-sized vessels with hyalinization were scantly present. On the basis of these findings, we diagnosed the lesion as mammary-type myofibroblastoma.
As mentioned above, mammary-type myofibroblastomas are rare mesenchymal neoplasms. The three previously published cases with a description of the radiographic features had occurred in the popliteal fossa, liver, and axilla (567). In these three cases, MRI showed a well-circumscribed mass with an intralesional fat component (567). Our case also showed similar MRI findings. Furthermore, Yoo et al. (10) reported a case of myofibroblastoma of the breast, which showed diffuse enhancement of the tumor on gadolinium-enhanced T1-weighted imaging and linear-shaped low signal intensity within the lesion on T1- and T2-weighted MRI. The present case showed similar MRI features in terms of the enhancement pattern and internal linear-shaped low signal intensity of the tumor. Thus, taken together, the MRI findings in our case could reflect the radiologic findings of either mammary-type myofibroblastoma or myofibroblastoma of the breast.
Although no study has been performed to verify the differences in imaging features between mammary-type myofibroblastoma and myofibroblastoma of the breast, it could be reasonable to presume that both show similar MRI features to the current case. In addition, in the current case, the tumor presented as a solitary, heterogeneous, and hyperechoic mass with some internal vascularity on USG. No demonstrable internal flow has been reported in mammary-type myofibroblastoma of the liver (6), whereas marked hypervascularity with tangled vascular flow was observed in myofibroblastoma of the breast (10). Because of the paucity of published literature describing the USG findings of mammary-type myofibroblastoma and myofibroblastoma of the breast, the vascular pattern of these tumors has not been extensively studied and it remains largely unclear. Because mammary-type myofibroblastomas can show various patterns of vascularity, further studies assessing the USG features of these tumors are required.
To the best of our knowledge, the case reported herein is the first case of mammary-type myofibroblastoma located in the buttock with descriptions of both USG and MRI findings. In the current case, the combination of these imaging studies was helpful in clarifying and understanding the components of the tumor, which contained both dense fibrous tissue and a fat component. On the basis of these radiologic findings, radiologists should consider lipomatous tumors, such as spindle cell lipoma, pleomorphic lipoma, myolipoma, angiolipoma, and liposarcoma, and fibrous tumors, such as cellular angiofibroma, fibromatosis, desmoplastic fibroblastoma, desmoid tumor, and malignant fibrous histiocytoma, in the differential diagnosis of palpable buttock masses. The important differential diagnosis of a mammary-type myofibroblastoma includes spindle cell lipoma, which may demonstrate a well-circumscribed complex fatty mass without adjacent fat stranding in the subcutis, and it may also show a variable amount of fat component in combination with nodular areas or soft tissue septations in the tumor. Spindle cell lipoma also shows a variable enhancement pattern, depending on the internal component of the tumor. A complex fatty mass in a middle-aged man, located in a typical site such as the posterior neck, suggests spindle cell lipoma rather than mammary-type myofibroblastoma. Another differential diagnosis of mammary-type myofibroblastoma includes cellular angiofibroma. The radiologic features of cellular angiofibroma show a mass with sharply defined margins showing variable heterogeneous high signal intensity on T2-weighted imaging and intense heterogeneous enhancement after gadolinium administration. Unfortunately, the imaging features of mammary-type myofibroblastomas are not specific (56710), and the mass shows overlapping radiologic findings with spindle cell lipoma and cellular angiofibroma. Therefore, due to the ambiguous appearance of these tumors on radiologic images, excisional biopsy is necessary to establish the exact diagnosis.
Previous studies have shown that mammary-type myofibroblastomas are rarely found in sites such as the axilla, retroperitoneum, mid-back, and buttock (2). Interestingly, previous studies have suggested that mammary-type myofibroblastomas are usually related to the embryonic milk-line (24). Our case was also thought to have originated from the embryonic milk-line, even though it involved the buttock. Notably, the clinical importance of mammary-type myofibroblastoma lies in its recognition as a definite benign neoplasm.
In conclusion, although involvement of the buttock is a very rare presentation of mammary-type myofibroblastoma, awareness of this rare entity is required for a better differential diagnosis of cases presenting as a well-circumscribed soft tissue mass with dense fibrotic tissue, an intratumoral fat component, and vascular structures.
Figures and Tables
Fig. 1
Mammary-type myofibroblastoma of the buttock in a 38-year-old woman.
A. Oblique plain pelvic radiograph showing a well-defined, oval, soft tissue mass in the left buttock.
B. Ultrasound scan showing a well-defined, echogenic mass with heterogeneous echotexture and variable posterior acoustic shadowing in the subcutaneous layer of the left buttock.
C. T1-weighted magnetic resonance image in the axial plane showing a hypointense mass with a small area of intralesional fat (arrow) in the left buttock.
D. T2-weighted image with fat saturation in the axial plane demonstrating a well-circumscribed mass with heterogeneous hyperintensity and internal
low signal intensity.
E. Axial contrast-enhanced fat-suppressed T1-weighted image showing heterogeneous enhancement of the mass with linear-shaped lesions showing low signal intensity. Vascular structures were noted in the peripheral aspect of the tumor.
F. Histology of the tumor shows fascicles of spindle cells with dense fibrous tissue and trapped adipose tissue on hematoxylin-eosin staining (original magnification, × 100).
G, H. The spindle cells show immunoreactivity for (G) cluster of differentiation 34 and (H) desmin (original magnification, × 100).
References
1. Wargotz ES, Weiss SW, Norris HJ. Myofibroblastoma of the breast. Sixteen cases of a distinctive benign mesenchymal tumor. Am J Surg Pathol. 1987; 11:493–502.
2. McMenamin ME, Fletcher CD. Mammary-type myofibroblastoma of soft tissue: a tumor closely related to spindle cell lipoma. Am J Surg Pathol. 2001; 25:1022–1029.
3. Bhullar JS, Varshney N, Dubay L. Intranodal palisaded myofibroblastoma: a review of the literature. Int J Surg Pathol. 2013; 21:337–341.
4. Abdul-Ghafar J, Ud Din N, Ahmad Z, Billings SD. Mammary-type myofibroblastoma of the right thigh: a case report and review of the literature. J Med Case Rep. 2015; 9:126.
5. Scotti C, Camnasio F, Rizzo N, Fontana F, De Cobelli F, Peretti GM, et al. Mammary-type myofibroblastoma of popliteal fossa. Skeletal Radiol. 2008; 37:549–553.
6. Millo NZ, Yee EU, Mortele KJ. Mammary-type myofibroblastoma of the liver: multi-modality imaging features with histopathologic correlation. Abdom Imaging. 2014; 39:482–487.
7. Agale SV, Warpe BM, Kumari G, Valand AG. Myofibroblastoma of axillary soft tissue in a child. J Cancer Tumor Int. 2015; 2:150–154.
8. Bigotti G, Coli A, Mottolese M, Di Filippo F. Selective location of palisaded myofibroblastoma with amianthoid fibres. J Clin Pathol. 1991; 44:761–764.
9. Magro G. Mammary myofibroblastoma: a tumor with a wide morphologic spectrum. Arch Pathol Lab Med. 2008; 132:1813–1820.
10. Yoo EY, Shin JH, Ko EY, Han BK, Oh YL. Myofibroblastoma of the female breast: mammographic, sonographic, and magnetic resonance imaging findings. J Ultrasound Med. 2010; 29:1833–1836.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download