Abstract
Purpose
To evaluate the short-term outcome of plug-assisted retrograde transvenous obliteration (PARTO) using vascular plugs and gelatin sponges in comparison with balloon-occluded retrograde transvenous obliteration (BRTO) for the management of gastric varices.
Materials and Methods
From January 2005 to October 2014, 171 patients were referred for management of gastric varices, of which, 52 patients with hemodynamically stable gastric varices (48 recent bleeding; 4 primary prophylaxes) were evaluated. Of these, 38 received BRTO (men/women 23/15; mean age 61.3; Child-Pugh classes A/B/C = 11/25/2) and 14 underwent PARTO (men/women 11/3; mean age 63.4; Child-Pugh classes A/B/C = 9/4/1). The technical success rate, complications, variceal changes, liver function, and exacerbation of ascites/pleural effusion were compared between the 2 groups within 3 months after the procedure.
Results
The technical success rates were 92.1% in the BRTO and 100% in the PARTO group. Procedure-related early complications occurred in the BRTO group alone (8%, n = 3). Among patients with technical success, follow-up CT at 1 month was available for 98% (n = 48/49). Complete thrombosis of gastric varices was achieved in 97.1% in the BRTO and 100% in the PARTO group. Worsening of esophageal varices was observed in 24% of the BRTO group alone (n = 8). The albumin level increased significantly in both groups and aspartate aminotransferase/alanine aminotransferase level improved significantly in the PARTO group (p < 0.05). Exacerbation of ascites/pleural effusion was observed in both groups (35.2% vs. 21.4%, both p > 0.05).
Upper gastrointestinal endoscopy is usually the first-line diagnostic and management tool for bleeding gastric varices (1). When endoscopy fails to control gastric variceal bleeding, a transjugular intrahepatic portosystemic shunt (TIPS) is recommended to decompress the portal circulation (12). However, TIPS may not be suitable for some patients with poor hepatic reserve, portal vein thrombosis, hepatic encephalopathy, or severe thrombocytopenia (123).
Balloon-occluded retrograde transvenous obliteration (BRTO) is an endovascular technique that was refined in Japan as a therapeutic adjunct or alternative to TIPS for management of gastric varices (4). The BRTO technique has been described in many reports (4567) and has shown considerable effectiveness with low rebleeding rates (45678910). Its advantages over TIPS include less invasiveness and greater performance ease in patients with poor hepatic reserve, encephalopathy, or refractory ascites (278). However, as BRTO requires an occlusion balloon catheter and sclerosing agents to occlude the portosystemic shunt, the indwelling catheter results in long procedural times and complications associated with the balloon (e.g., balloon rupture) (111213). Ethanolamine oleate (EO), the traditional sclerosing agent used in Japan and Korea, is associated with serious complications, such as pulmonary edema, disseminated intravascular coagulation, portal vein thrombosis, severe renal dysfunction, and anaphylactic reactions (51213). In the absence of adequate EO concentrations and antidotes, 3% sodium tetradecyl sulfate (STS) has been used as the sclerosant of choice in the United States (14). The sclerosing effect of STS foam is theoretically more rapid than EO, but problems associated with balloon catheter remain.
Gwon et al. (15) reported a variant of BRTO, vascular plugassisted retrograde transvenous obliteration (PARTO), to overcome the drawbacks of BRTO using sclerosant by PARTO is an alternative endovascular treatment that uses a vascular plug and gelatin sponge particles instead of balloon occlusion of the gastrorenal shunt and a sclerosing agent. However, gelatin sponge is an absorbable agent that produces a temporary occlusion when used for gastric variceal embolization, leading to a greater likelihood of recanalization, as compared with a permanent agent such as EO.
The purpose of this study was to evaluate the technical feasibility, short-term effects, and complications of PARTO using a vascular plug with gelatin sponge particles, and compare it with BRTO using an occlusion balloon with a permanent sclerosant for management of gastric varices.
From January 2005 to October 2014, 171 patients with recent gastric variceal bleeding or with high risk of bleeding on endoscopy were referred for management. Institutional Review Board approval was obtained for this retrospective study. The diagram of inclusion and exclusion criteria for gastric variceal management was shown in Fig. 1. A total 107 patients with emergent bleeding gastric varices were treated with TIPS; 64 patients with stable gastric varices were evaluated with preprocedural contrast-enhanced computed tomography (CT). Dynamic CT was performed using multidetector CT scanners (Somatom Definition Flash, Siemens Medical Solutions, Forchheim, Germany or Aquilion One, Toshiba, Tokyo, Japan). Using a bolus tracking technique, the arterial phase was performed 20 seconds after 100 above baseline Hounsfield units was attained at the abdominal aorta. The portal phase images were obtained with a scan delay of 30 seconds after the end of previous arterial phase. Three-dimensional (3D) volume rendering CT images were obtained by contrast-enhanced CT (TeraRecon, San Mateo, CA, USA) to analyze the presence and size of the gastrorenal shunt, complex collateral vessels, and venous drainage pattern (Fig. 2). Gastric varices were classified according to the venous drainage pattern into types A, B, C, or D according to criteria of Sabri and Saad (16). According to 3D volume rendering CT images, type A and type D gastric varices with a shunt diameter over 18 mm, were treated with TIPS. Patients with prominent secondary shunting (type C) were included in BRTO but excluded from PARTO. In total, 52 patients were reviewed retrospectively in this study.
Detailed demographics were presented in Table 1. Among 52 patients, 48 had a history of bleeding within 2 weeks, with or without endoscopic treatment (36 in the BRTO group; 12 in the PARTO group), and the other 4 had no history of variceal bleeding (2 patients in each group). These 4 patients had gastric varices with a risk of rupture on endoscopic examination (1718).
The evaluation of variceal changes on endoscopy was based on the endoscopic criteria of Japan; variceal changes were classified by form and red color sign (1718) as tortuous (F1), nodular (F2), or tumorous (F3).
Thirty-eight patients (23 men, 15 women; mean age 61.3, range 42–83 years) underwent BRTO from January 2005 to December 2011; the Child-Pugh class was A in 11 patients, B in 25, and C in 2. Beginning in January 2012, BRTO had to be changed to PARTO because EO became unavailable. Fourteen patients (11 men, 3 women; mean age 63.4, range 49–79 years) underwent PARTO from January 2012 to October 2014; the Child-Pugh class was A in 9 patients, B in 4, and C in 1. Esophageal varices were observed on preprocedural CT or endoscopy in 13 patients (34%) before BRTO and 5 (36%) before PARTO.
All patients were hemodynamically stable at the time of the procedure. Under conscious sedation, a 9 Fr Flexor® Ansel guiding sheath (Cook, Bloomington, IN, USA) was introduced through the right femoral vein and placed in the proximal aspect of the gastrorenal shunt for stable access. Using a 4 Fr Cobra catheter (Terumo, Tokyo, Japan), the efferent aspect of the gastrorenal shunt was accessed coaxially. Among various types of vascular plug, we used Amplatzer vascular plug II (AGA Medical, Golden Valley, MN, USA). The vascular plug was deployed halfway at the narrowest region of the gastrorenal shunt through the 9 Fr sheath (Fig. 3A). The size of the selected vascular plug was approximately 20% larger than the narrowest diameter of the gastrorenal shunt, as measured on CT images. After positioning and partly deploying the vascular plug, a variceal/shunt venogram was performed with the Cobra catheter, which was advanced proximally into the gastric varices or as close as possible (Fig. 3B). Gelfoam sheets were cut with scissors (1–8 mm3), and gelfoam and contrast agents were mixed into a slurry by sequential mixing through a 3-way stopcock connected to 2 syringes. Gelfoam slurry was injected through the Cobra catheter to achieve filling and stasis within the entire efferent shunt and varices. In some type B patients, leaking collateral veins, such as the inferior phrenic or paravertebral veins, were opacified. Microcatheters were advanced into the collateral veins using triple coaxial method and these veins were occluded using gelfoam pledgets through a microcatheter.
The endpoint of gelatin sponge infusion (embolization) was when the gastric varices were filled completely and/or afferent vasculature was filled minimally on fluoroscopy. Post-embolization venography using the Cobra catheter was performed again to confirm complete occlusion of the efferent shunt and complete stasis within the varices (Fig. 3C). On confirmed completion, the vascular plug was deployed completely and detached (Fig. 3D).
Contrast-enhanced CT was performed 1 month after the procedure to evaluate thrombosis of the gastrorenal shunt and gastric varices, and changes in the esophageal varices. Changes in esophageal varices were evaluated on CT images (19) with or without upper endoscopy, which was performed by the referring clinicians. Follow-up clinical and laboratory tests were performed, and exacerbation of ascites or pleural effusion was evaluated at 1 and 3 months after BRTO or PARTO.
Technical success was defined as successful placement of the vascular plug and stasis of contrast medium and complete cessation of flow within the gastric varices on fluoroscopy immediately after the procedure. Early complications were defined as those that occurred within the first 4 weeks after the procedure, and delayed complications were those that occurred later. Complete thrombosis of the varices was defined as complete clotting of the targeted varices, as seen on follow-up CT images in the portal phase within 1 month. Complete thrombosis was defined as disappearance of enhancement of the intraluminal and intramural portions of the gastric varices. Partial thrombosis was defined as residual enhancement within the intraluminal or intramural portion of the gastric varices. The evaluation of esophageal varices on CT was classified according to Yu et al. (19) as: 1) definitely no esophageal varices; 2) likely no esophageal varices; 3) probably esophageal varices; 4) likely esophageal varices; or 5) definitely esophageal varices. New appearance or increases in ascites on comparison of CT images, or symptomatic abdominal distension, were judged as exacerbations. Exacerbation of pleural effusion was evaluated by follow-up CT images and chest radiography. The significance of differences in demographics was analyzed using the two-sample t-test, Mann-Whitney test, and χ2-test. The differences in procedural time were analyzed using the Mann-Whitney test. The repeated analysis of variance test was used to compare serial laboratory values between the 2 groups before and after the procedure. Cross-tabulation analysis was performed to analyze the association between the postprocedural exacerbation of ascites/pleural effusion and the Child-Pugh class. All statistical analyses were performed using SPSS version 22.0 software (IBM SPSS, Armonk, NY, USA), with p-values < 0.05 considered statistically significant.
The technical success rate was 92.1% (n = 35/38) in the BRTO group and 100% (n = 14/14) in the PARTO group. The causes of technical failure in the BRTO group were retroperitoneal rupture of the gastrorenal shunt that led to the cessation of the procedure (n = 1), inability to complete the procedure due to the lack of patient's cooperation attributable to underlying encephalopathy (n = 1), and repeated balloon-occlusion catheter ruptures during EO instillation (n = 1). Of these 3 patients, the first 2 refused emergent TIPS conversion and died of rebleeding within 24 hours. The third patient refused TIPS conversion, but showed partial thrombosis of gastric varices on follow-up CT with no variceal bleeding, and was excluded from analysis.
In the PARTO group, a single vascular plug was sufficient to occlude gastrorenal shunts in all patients: 8 mm diameter (n = 1); 10 mm (n = 2); 12 mm (n = 9); 14 mm (n = 1); and 16 mm (n = 1). The microcatheter was used for more effective embolization of the collateral vessels in 12 patients.
In the BRTO group, the mean procedure time including the balloon dwell time from venous access to the end of the procedure was 398 min (range, 85–1080), and the mean procedure time excluding the balloon dwell time was 143 min (range, 70–250). In the PARTO group, the mean procedure time was 78 min (range, 60–120) (p = 0.001).
Follow-up CT at 1 month was available for 98% of the patients who had technically successful procedures (n = 48/49). Complete thrombosis of both the gastric varices and gastrorenal shunt (Fig. 4) was observed in 97.1% (n = 33/34) of the patients who received BRTO, and in 100% (n = 14/14) of the patients who underwent PARTO. One patient in the BRTO group showed partial thrombosis of the gastric varices and gastrorenal shunt. Although he had a history of recent variceal bleeding before BRTO, there was no rebleeding during the follow-up period. Furthermore, the regrowth of gastric varices was not observed on follow-up endoscopy, and no additional treatment was performed in this patient. Together with CT imaging, follow-up endoscopy was performed within 3 months by the referring clinicians in 20 patients (53%) in the BRTO group, and 10 patients (72%) in the PARTO group. The mean follow-up periods after BRTO and PARTO were 72 (range, 10–90) and 86 days (range, 59–90), respectively. Follow-up endoscopy showed marked shrinkage or disappearance of the gastric varices in all 30 patients. No significant differences were noted on follow-up CT for esophageal varices at 1 month in both groups. Follow-up endoscopy in the BRTO group revealed some aggravation of the preexisting esophageal varices in 5 patients, and newly developed esophageal varices in 3 patients. In the BRTO group, tortuous varices (F1) changed to the nodular type (F2) in 4 patients, and the nodular type (F2) changed to the tumorous type (F3) in 1 patient. In the PARTO group, varices were nodular (F2) in 4 patients and tortuous (F1) in 1 patient; no changes were observed. However, there were no cases of variceal bleeding during either of the follow-up periods, and none of the patients received treatment. Table 2 showed the short-term results following BRTO and PARTO.
None of the patients in the PARTO group showed procedurerelated early complications, as compared to 8% of the patients (n = 3/38) in the BRTO group. As mentioned above, 2 patients with technical failure died of variceal bleeding. One patient with technical success had recurrent bleeding and died within 24 hours after BRTO. In this patient, TIPS conversion could not be performed due to acute deterioration of vital signs, which left no time for intervention.
Forty-eight patients had laboratory measurements available at the 1- and 3-month follow-up. As compared with preprocedural laboratory values, there were no significant changes in the levels of total bilirubin, or prothrombin time-international normalized ratio (based on INR) at either follow-up. However, the serial mean serum albumin level increased significantly from 3.14 ± 0.49 to 3.76 ± 0.47 g/dL in the BRTO group (p = 0.000), and from 3.54 ± 0.62 to 3.87 ± 0.50 g/dL in the PARTO group (p = 0.023). Similarly, the aspartate aminotransferase (AST) and the alanine aminotransferase (ALT) level at 3 months significantly improved from 49.6 ± 29.01 to 32.7 ± 9.52 IU/L (p = 0.042) and from 20.8 ± 9.07 to 17.0 ± 5.85 IU/L (p = 0.039), respectively, in the PARTO group (Table 3).
The exacerbation of ascites/pleural effusion after BRTO or PARTO was evaluated in 48 patients with technical success. At 3 months, the cumulative incidence was 35.2% (n = 12/34) after BRTO and 21.4% (n = 3/14) after PARTO. Neither pleural effusion nor ascites were present before the procedure in any of the patients. On cross-tabulation analysis, the association between the incidence of ascites/pleural effusion and the Child-Pugh class was not significant in either group (both p > 0.05) (Table 4).
BRTO is an established procedure in Japan and Korea, with considerable effectiveness in controlling gastric variceal bleeding with low rebleeding rates (456789). However, BRTO requires prolonged indwelling of an occlusion balloon catheter, which makes this procedure difficult to tolerate in long-term bed-ridden patients. A permanent sclerosant can completely eradicate a gastric variceal complex, although this would increase portal hypertension (21219). Therefore, it is important to determine whether vascular plug-assisted gelatin sponge embolization could eradicate gastric varices as effectively as a permanent sclerosant.
Although the PARTO procedure (15) is less complex than BRTO, PARTO does not allow complete variceal opacification during the procedure. Gelatin sponge particles used as embolic material to obtain stasis of venous flow may not achieve complete obliteration of variceal complexes, and this may later result in recanalization of the varices or collaterals. Hence, it is uncertain whether PARTO is as good as BRTO due to the possibility of recanalization of previously occluded varices.
Gwon et al. (15) reported that follow-up CT within 1 week after PARTO showed complete thrombosis of both gastric varices and gastrorenal shunts in all patients, and a 2-month follow-up CT showed complete obliteration. Our study also demonstrated complete occlusion of the gastric varices and gastrorenal shunts in all 14 patients who underwent PARTO, which was confirmed by the 1-month follow-up CT.
We encountered 3 cases of BRTO technical failure. Two of these were related to the occlusion balloon catheter (long-term indwelling, balloon rupture during procedure) and the third to retroperitoneal rupture of the gastrorenal shunt during the procedure. In one case of failure of the long-term indwelling catheter, we could not complete BRTO because of the lack of patient cooperation due to serious hepatic encephalopathy. The other cases could have been converted to TIPS immediately, but patients with held consent. In contrast, there were no issues related to the occlusion device in the PARTO group.
The use of gelatin sponge particles as embolic material is of most concern in PARTO. Resorption of these agents causes temporary occlusion, and subsequent recanalization can occur within a few weeks (15). Lubarsky et al. (20) reported that vessels embolized with gelfoam typically recanalize in several weeks. As vessel embolization with thrombosis may be organized in 3 months, a 3-month follow-up was deemed sufficient for the analysis of short-term results. In the present study, there was complete thrombosis of gastric varices and gastrorenal shunts at 1 month after PARTO, and none of the patients showed recurrent variceal vessels at 3 months.
Although gelatin sponges could reportedly trigger a cascade of hypercoagulopathy, which may lead to portal venous thrombosis or systemic embolism (112122), no such complications were observed in our study.
In BRTO, an indwelling balloon is necessary to prevent the influx of EO into the systemic circulation, and ensure that the sclerosant passes through the shunt to the variceal complex, so that intraluminal thrombosis with sclerosis reaches completion. The indwelling time of the occlusion balloon varies from 30 min to overnight (7152023). Prolonged indwelling of the occlusion balloon may lead to potential problems, such as balloon rupture, infection, and bleeding, as well as patient inconveniences, such as prolonged bed-ridden periods with immobilization. In our study, the mean procedural time for BRTO (not including the indwelling balloon catheter time) was 143 min (range, 70–250), whereas that of PARTO was 78 min (range, 60–120). Because PARTO does not require an indwelling balloon catheter or a sclerosing agent, it has significantly decreased procedure and fluoroscopy time. Despite the common purpose of PARTO and BRTO, PARTO seems to be technically easier, safer, and more convenient and comfortable for patients, with less radiation exposure.
The presence of multiple collateral vessels of gastric varices is one of the most challenging situations in PARTO, because these vessels are not fully visualized until the gastric varices are filled with gelatin sponge particles. Patient selection and collateral vessels embolization following visualization of collateral vessels is often challenging. To avoid this potential problem, patients with prominent secondary shunts (type C) detected on preprocedural CT were not considered as candidates for PARTO in our study. Furthermore, small collaterals and portosystemic shunts can be more difficult to assess fully by imaging (7). These small collaterals were embolized during the procedure using triple coaxial microcatheter selection.
Gastric varices were thrombosed successfully in both groups, except in 2 patients in the BRTO group who had technical failures; they had refused the TIPS procedure after BRTO failure.
Obliteration of the gastrorenal shunt may worsen esophageal varices after the procedure due to the redirection of portal flow from the gastric varices. Previous studies have reported that the worsening rate of esophageal varices after BRTO varied from 10% to 67% (51320). In our study, follow-up CT and endoscopy revealed that 8 patients (24%) in the BRTO group showed worsening of esophageal varices (n = 5), or had newly developed varices (n = 3). Although the sensitivity for detecting small esophageal varices with CT may be low, none of the patients in the PARTO group experienced worsening of esophageal varices during the follow-up period. Permanent sclerosis of the gastric variceal complex was considered likely to increase the chance of portal redirection. Although follow-up CT of the PARTO patients showed complete thrombosis of the gastric variceal complex, unrevealed microshunts or recanalized veins can exist in the case of gelfoam-embolized PARTO. BRTO provides added benefits by maintaining more favorable hemodynamics because of the preservation of liver function, as compared to TIPS (1112). Gwon et al. (15) reported an improvement in the Child-Pugh class in 12 of 18 patients (67%), and a significant decrease in the INR 1 month after PARTO. Gwon et al. (24) reported improvement in Child-Pugh score in 24 patients (40%) at 1-month follow-up in a prospective multicenter study. In our patients, significant improvements in albumin (p = 0.003) levels were observed in both groups. Furthermore, there were significant changes in AST and ALT level within 3 months after the procedure in the PARTO group, suggesting that PARTO, like BRTO, may be effective in improving liver function. In general, BRTO can influence portal hemodynamics because it occludes major portosystemic shunts, and increases the portal hepatic blood flow and portal pressure. The increased pressure of the portal blood flow may subsequently induce a high incidence of ascites and pleural effusion (2526). In fact, several factors influence the incidence of both, such as a high Child-Pugh class, concomitant existence of another disease such as hepatocellular carcinoma, and hypoalbuminemia. In our study, the incidence of postprocedural ascites or pleural effusion was lower in the PARTO group than in the BRTO group (21.4% vs. 35.2%). The BRTO group showed a higher number of Child-Pugh classes B and C than the PARTO group. Cross-tabulation analysis to correct for the effect of the Child-Pugh class on the incidence of ascites or pleural effusion showed no significant association between the Child-Pugh classes and exacerbation of ascites/pleural effusion (p > 0.05). Therefore, the higher incidence of postprocedural ascites or pleural effusion in the BRTO group was possibly not associated with the Child-Pugh class. We hypothesize that BRTO using a sclerosant can induce more complete obstruction of the gastrorenal shunts and collateral efferent veins in comparison with PARTO, or that PARTO may induce incomplete obstruction of the gastric varices or microshunts, or microrecanalization of thrombosed veins. This would result in a high incidence of ascites/pleural effusion in the BRTO group.
In conclusion, PARTO can reduce procedural time and has a high technical success rate, as compared with BRTO. There were no procedure-related complications in any patients who underwent PARTO. Post-procedural hepatic function restoration was as effective as after BRTO. Hence, PARTO is technically feasible and safe and seems to be equivalent to or better than BRTO for management of gastric varices, with good short-term results.
The limitations of this study included its retrospective design and small sample size, which might limit the generalizability of the findings. The duration of the follow-up is also critical. A prospective long-term follow-up study with a larger number of patients will be needed.
Figures and Tables
 | Fig. 1Schematic diagram for BRTO/PARTO management. Solid arrows: from 2005 to 2011. Dashed arrows: from 2012 to 2014. The types of gastric varices were classified using 3D volume rendering images based on the criteria of Sabri and Saad (16). *Gastric variceal bleeding within 2 weeks, †GR shunt: gastrorenal shunt.BRTO = balloon-occluded retrograde transvenous obliteration, PARTO = plug-assisted retrograde transvenous obliteration, TIPS = transjugular intrahepatic portosystemic shunt, ø = largest diameter of gastrorenal shunt
|
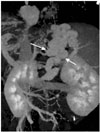 | Fig. 2Preprocedural evaluation of PARTO in a 49-year-old man. The 3D volume rendering image provides clear visualization of the gastrorenal shunt (white arrows) and collateral vessels, as well as the presence and size of the gastrorenal shunt and venous drainage pattern.PARTO = plug-assisted retrograde transvenous obliteration
|
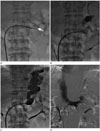 | Fig. 3PARTO procedure (the same patient as in Fig. 2).
A. After a 9 Fr vascular sheath was placed in the gastrorenal shunt and the efferent shunt was accessed coaxially using a 4 Fr Cobra catheter, the vascular plug was deployed halfway at the narrowest region of the gastrorenal shunt through the vascular sheath (white arrow).
B. The efferent shunt was evaluated by advancing the catheter proximally into or close to the gastric varices using a coaxial catheter technique.
C. After embolization of the gastrorenal shunt using gelfoam particle, a static fluoroscopic view showed stasis of the contrast material and gelfoam within the varices and gastrorenal shunt. Then, the vascular plug was deployed completely and detached.
D. Final venography through the vascular sheath showed complete occlusion of the gastrorenal shunt without backflow from the gastric varices.
PARTO = plug-assisted retrograde transvenous obliteration
|
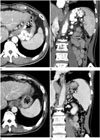 | Fig. 4Pre- and postprocedural CT images of PARTO (the same patient as in Figs. 2 and 3).
A, B. Preprocedural axial (A) and coronal (B) CT scan images show prominent fundal gastric varices and a gastrorenal shunt.
C, D. Axial (C) and coronal (D) CT images 1 month after PARTO show complete obliteration of the targeted varices.
PARTO = plug-assisted retrograde transvenous obliteration
|
Table 1
Patient Demographics (n = 52)
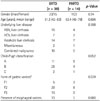
Table 2
Short-Term Outcomes and Complications after BRTO and PARTO

Table 3
Differences in Liver Function Parameters within One and Three Months

*Data are presented as the means ± standard deviations.
ALT = alanine aminotransferase, AST = aspartate aminotransferase, BRTO = balloon-occluded retrograde transvenous obliteration, INR = international normalized ratio, PARTO = plug-assisted retrograde transvenous obliteration, PT = prothrombin time
Table 4
Comparison of the Incidence of Ascites or Pleural Effusion and the Child-Pugh Classification

References
1. Al-Osaimi AM, Caldwell SH. Medical and endoscopic management of gastric varices. Semin Intervent Radiol. 2011; 28:273–282.
2. Saad WE, Darcy MD. Transjugular intrahepatic portosystemic shunt (TIPS) versus balloon-occluded retrograde transvenous obliteration (BRTO) for the management of gastric varices. Semin Intervent Radiol. 2011; 28:339–349.
3. Ripamonti R, Ferral H, Alonzo M, Patel NH. Transjugular intrahepatic portosystemic shunt-related complications and practical solutions. Semin Intervent Radiol. 2006; 23:165–176.
4. Kanagawa H, Mima S, Kouyama H, Gotoh K, Uchida T, Okuda K. Treatment of gastric fundal varices by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol. 1996; 11:51–58.
5. Fukuda T, Hirota S, Sugimura K. Long-term results of balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy. J Vasc Interv Radiol. 2001; 12:327–336.
6. Hirota S, Matsumoto S, Tomita M, Sako M, Kono M. Retrograde transvenous obliteration of gastric varices. Radiology. 1999; 211:349–356.
7. Saad WE. Balloon-occluded retrograde transvenous obliteration of gastric varices: concept, basic techniques, and outcomes. Semin Intervent Radiol. 2012; 29:118–128.
8. Fukuda T, Hirota S, Matsumoto S, Sugimoto K, Fujii M, Tsurusaki M, et al. Application of balloon-occluded retrograde transvenous obliteration to gastric varices complicating refractory ascites. Cardiovasc Intervent Radiol. 2004; 27:64–67.
9. An EJ, Kim YH, Kim SH. Comparison of coil embolization and sclerotherapy of collateral veins during balloon occluded retrograde transvenous obliteration: its long term effect for gastric varix treatment. J Korean Soc Radiol. 2011; 65:357–363.
10. Kim JH, Kim YH, An EJ, Kim SH, Choi JS. CT findings after BRTO in patients with gastric varix bleeding: can we predict varix recurrence. J Korean Soc Radiol. 2011; 64:131–137.
11. Park SJ, Chung JW, Kim HC, Jae HJ, Park JH. The prevalence, risk factors, and clinical outcome of balloon rupture in balloon-occluded retrograde transvenous obliteration of gastric varices. J Vasc Interv Radiol. 2010; 21:503–507.
12. Hiraga N, Aikata H, Takaki S, Kodama H, Shirakawa H, Imamura M, et al. The long-term outcome of patients with bleeding gastric varices after balloon-occluded retrograde transvenous obliteration. J Gastroenterol. 2007; 42:663–672.
13. Chikamori F, Kuniyoshi N, Shibuya S, Takase Y. Eight years of experience with transjugular retrograde obliteration for gastric varices with gastrorenal shunts. Surgery. 2001; 129:414–420.
14. Sabri SS, Swee W, Turba UC, Saad WE, Park AW, Al-Osaimi AM, et al. Bleeding gastric varices obliteration with balloonoccluded retrograde transvenous obliteration using sodium tetradecyl sulfate foam. J Vasc Interv Radiol. 2011; 22:309–316. quiz 316.
15. Gwon DI, Ko GY, Yoon HK, Sung KB, Kim JH, Shin JH, et al. Gastric varices and hepatic encephalopathy: treatment with vascular plug and gelatin sponge-assisted retrograde transvenous obliteration--a primary report. Radiology. 2013; 268:281–287.
16. Sabri SS, Saad WE. Balloon-occluded retrograde transvenous obliteration (BRTO): technique and intraprocedural imaging. Semin Intervent Radiol. 2011; 28:303–313.
17. Hashizume M, Kitano S, Yamaga H, Koyanagi N, Sugimachi K. Endoscopic classification of gastric varices. Gastrointest Endosc. 1990; 36:276–280.
18. Tajiri T, Yoshida H, Obara K, Onji M, Kage M, Kitano S, et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Dig Endosc. 2010; 22:1–9.
19. Yu NC, Margolis D, Hsu M, Raman SS, Lu DS. Detection and grading of esophageal varices on liver CT: comparison of standard and thin-section multiplanar reconstructions in diagnostic accuracy. AJR Am J Roentgenol. 2011; 197:643–649.
20. Lubarsky M, Ray CE, Funaki B. Embolization agents-which one should be used when? Part 1: large-vessel embolization. Semin Intervent Radiol. 2009; 26:352–357.
21. Choi SY, Won JY, Kim KA, Lee do Y, Lee KH. Foam sclerotherapy using polidocanol for balloon-occluded retrograde transvenous obliteration (BRTO). Eur Radiol. 2011; 21:122–129.
22. Miura H, Yamagami T, Tanaka O, Yoshimatsu R. Portopulmonary venous anastomosis detected at balloon-occluded retrograde transvenous obliteration for gastric varices. J Vasc Interv Radiol. 2013; 24:131–134.
23. Koito K, Namieno T, Nagakawa T, Morita K. Balloon-occluded retrograde transvenous obliteration for gastric varices with gastrorenal or gastrocaval collaterals. AJR Am J Roentgenol. 1996; 167:1317–1320.
24. Gwon DI, Kim YH, Ko GY, Kim JW, Ko HK, Kim JH, et al. Vascular plug-assisted retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy: a prospective multicenter study. J Vasc Interv Radiol. 2015; 26:1589–1595.
25. Chikamori F, Kuniyoshi N, Shibuya S, Takase Y. Short-term hemodynamic effects of transjugular retrograde obliteration of gastric varices with gastrorenal shunt. Dig Surg. 2000; 17:332–336.
26. Watanabe M, Shiozawa K, Ikehara T, Nakano S, Kougame M, Otsuka T, et al. Short-term effects and early complications of balloon-occluded retrograde transvenous obliteration for gastric varices. ISRN Gastroenterol. 2012; 2012:919371.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download