Abstract
Charcoal remains stable without causing a foreign body reaction and it may be used for preoperative localization of a non-palpable breast mass. However, cases of post-charcoal-marking granuloma have only rarely been reported in the breast, and a charcoal granuloma can be misdiagnosed as malignancy. Herein, we report the ultrasound, computed tomography (CT), 18F-fluorodeoxyglucose-positron emission tomography/CT, and breast-specific gamma imaging findings of recurrence-mimicking charcoal granuloma after breast conserving surgery, following localization with charcoal in a breast cancer patient.
Charcoal is used for localization of a non-palpable breast mass. Although charcoal is known to be safe when injected subcutaneously for preoperative localization, it can cause a foreign body reaction (1). Several cases of post-charcoal-marking granuloma of the breast have been reported, but the imaging modalities employed were limited to mammography or ultrasonography (23) and other imaging findings have not yet been reported.
Herein, we report multimodal imaging [i.e., ultrasound (US), computed tomography (CT), 18F-fluorodeoxyglucose (FDG)-positron emission tomography/CT (PET/CT), and breast-specific gamma imaging (BSGI)] findings of a recurrence-mimicking charcoal granuloma in a breast cancer patient.
A 60-year-old woman visited our hospital for a routine breast exam. She had no palpable lesion in the breast. However, a 0.9 cm mass with an indistinct margin was detected in the left lower outer quadrant of the breast by mammography (Fig. 1A). US showed a 0.8 cm hypoechoic mass with an indistinct margin and a subtle irregular shape at the 4 o'clock position in the left breast, which was classified as Breast Imaging Reporting And Data System 4 (Fig. 1B). Because she was aware of the presence of a previous, probably benign mass in her breast, she opted for excision of the mass. Therefore, preoperative US-guided localization was performed using charcoal. A mixture of 1 mL charcoal (Charcotrace; Phebra, Sydney, Australia) and 1 mL normal saline was injected around the mass and the anterior fat layer. The postoperative pathologic result was invasive ductal carcinoma with a positive margin. The surgeon decided to perform reoperation for breast conserving surgery and a second US-guided charcoal localization was performed, mainly in the subcutaneous fat layer of the postoperative field, on the morning of the surgery. The cancer was removed successfully and a 4 mm residual ductal carcinoma in situ with a negative margin remained behind. After surgery, the patient received chemotherapy (adriamycin and cyclophosphamide) and radiotherapy (up to 59.4 Gy) to the left breast.
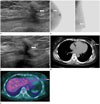
On the first 6-month follow-up US after surgery, a spiculated irregular hypoechoic lesion was detected in the left middle outer quadrant of the breast (Fig. 2A) and the possibility of a prominent postoperative scar was considered. Mammography showed no abnormal findings, except for postoperative distortion of the left breast. On BSGI, intense focal uptake in the left middle outer quadrant of the breast was observed, and the possibility of postoperative change or recurrent lesion was considered (Fig. 2B).
On the second follow-up US performed 12 months after surgery, significant change was not detected in the spiculated irregular hypoechoic lesion in the left breast (Fig. 2C). Chest CT showed that the lesion was an ovoid hyperdense (47–66 Hounsfield units) mass with adjacent postoperative distortion, which did not significantly enhance after contrast administration (Fig. 2D). On FDG-PET/CT, a newly developed hypermetabolic lesion (maximum standardized uptake value = 2.6–3.7) was noted in the previous operative bed in the left middle outer quadrant of the breast (Fig. 2E). Based on the suspicion of recurrence, a US-guided biopsy was performed using a 14-gauge semi-automated core biopsy needle (Stericut; TSK, Tochigi, Japan). The gross specimens were dark-pigmented soft-tissue fragments (Fig. 3A). Histologic evaluation revealed extensive charcoal deposits and multinucleated giant cells with surrounding granulomatous inflammation, but no malignant cells (Fig. 3B).
The patient's follow-up US, PET/CT, and BSGI over the course of three years revealed no significant interval change in the lesion.
Several cases of malignancy-mimicking granuloma of the breast following charcoal marking have been reported, but only mammographic or US findings have been reported (23). In contrast, our case was supported by US, chest CT, FDG-PET/CT, and BSGI findings. The US findings of charcoal granuloma in our case included a spiculated and irregular hypoechoic lesion without an interval change, which are consistent with those of a previously reported charcoal granuloma of the breast (3). Although the size of the lesion was not helpful in differentiating recurrence from a benign postoperative change, the lack of change was an important clue. FDG-PET/CT revealed hypermetabolism of the charcoal granuloma. A charcoal granuloma can present as a hypermetabolic lesion due to FDG uptake by numerous activated multinucleated giant cells (45). However, our lesion showed high attenuation on precontrast CT scan, with no definite enhancement on postcontrast scans. High attenuation of the lesion can be due to the presence of highly dense activated carbon particles (4). The PET/CT and CT findings were in accordance with those of previously reported charcoal granulomas in the peritoneal cavity after intraperitoneal chemotherapy with the use of charcoal, and in the postoperative neck (456).
However, to the best of our knowledge, BSGI findings of a charcoal granuloma have not yet been reported. BSGI is a radioisotopic imaging modality that is emerging as a useful complementary tool to diagnose breast cancer when more information is needed after questionable initial radiological findings (7). BSGI is based on the increased uptake of 99mTc-sestamibi in cancer cells compared with that in normal breast tissue, and the difference in uptake is thought to be due to increased vascularity and mitochondrial activity in cancer cells (8). Our BSGI results showed intense focal uptake in a malignancy-mimicking charcoal granuloma. Indeed, inflammatory lesions, as in the present case, are one of the well-known causes of false positive 99mTc-sestamibi uptake (9).
Charcoal is usually used to localize a non-palpable breast mass and is a biologically inert material when injected subcutaneously, but it can remain in situ for up to 60 days without causing a foreign body reaction (1). However, an experimental study reported that charcoal particles are ingested by macrophages and are very slowly carried away from the injection site, causing inflammation (10). In fact, charcoal remaining in the surgical bed could facilitate an inflammatory reaction during postoperative healing. In the present case, our patient was injected with charcoal twice (> 1 mL) and received radiotherapy after surgery, possibly resulting in a synergistic effect leading to the charcoal granuloma (6).
It is difficult to distinguish tumor recurrence from a benign lesion such as a postoperative change or charcoal granuloma using US, FDG-PET/CT, CT or BSGI alone. CT can be somewhat helpful due to hyperattenuation of a charcoal granuloma. A US-guided core-needle biopsy can be effective in obtaining a better diagnosis.
In summary, we reported US, CT, FDG-PET/CT and BSGI findings of a charcoal granuloma discovered in the breast after breast-conserving surgery. Charcoal granuloma should be included in the differential diagnosis of incidentally detected lesions at the site of preoperative localization using charcoal.
Figures and Tables
Fig. 1
Preoperative mammography and breast ultrasound of a 60-year-old woman.
A. Mammography reveals a 0.9 cm mass (arrows) with an indistinct margin in the left lower outer quadrant of the breast.
B. Breast ultrasound shows a 0.8 cm hypoechoic mass (arrow) with an indistinct margin and a subtle irregular shape at the 4 o'clock position in the left breast.
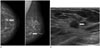
Fig. 2
BSGI, US, FDG-PET/CT, and chest CT findings of a charcoal granuloma.
A. The first 6-month follow-up US after surgery demonstrates a spiculated irregular hypoechoic lesion (arrow) in the left middle outer quadrant of the breast.
B. The first BSGI reveals intense focal uptake (arrows) in the left middle outer quadrant of the breast and it was considered as a postoperative change or recurrent lesion.
C. The second 12-month follow-up US after surgery shows no significant change in the spiculated irregular hypoechoic lesion (arrow) in the left breast.
D. Chest CT shows that the lesion is an ovoid hyperdense mass (47–66 Hounsfield units) (arrow) with adjacent postoperative distortion and it is not significantly enhanced after contrast administration.
E. FDG-PET/CT shows a hypermetabolic lesion (arrow) (SUVmax = 2.6–3.7) in the previous operative bed of the left middle outer quadrant of the breast.
BSGI = breast-specific gamma imaging, FDG-PET/CT = 18F-fluorodeoxyglucose-positron emission tomography/computed tomography, SUVmax = maximum standardized uptake value, US = ultrasound
References
1. Langlois SL, Carter ML. Carbon localisation of impalpable mammographic abnormalities. Australas Radiol. 1991; 35:237–241.
2. Patrikeos A, Wylie EJ, Bourke A, Frost F. Imaging of carbon granulomas of the breast following carbon track localization. Clin Radiol. 1998; 53:845–848.
3. Ruiz-Delgado ML, López-Ruiz JA, Sáiz-López A. Abnormal mammography and sonography associated with foreign-body giant-cell reaction after stereotactic vacuum-assisted breast biopsy with carbon marking. Acta Radiol. 2008; 49:1112–1118.
4. Kim YK, Park HS. Foreign body granuloma of activated charcoal. Abdom Imaging. 2008; 33:94–97.
5. Lim ST, Jeong HJ, Kim DW, Yim CY, Sohn MH. F-18 FDG PET-CT findings of intraperitoneal carbon particles-induced granulomas mimicking peritoneal carcinomatosis. Clin Nucl Med. 2008; 33:321–324.
6. Choi JW, Moon WJ, Choi N, Roh HG, Kim MY, Kim NR, et al. Charcoal-induced granuloma that mimicked a nodal metastasis on ultrasonography and FDG-PET/CT after neck dissection. Korean J Radiol. 2015; 16:196–200.
7. Brem RF, Floerke AC, Rapelyea JA, Teal C, Kelly T, Mathur V. Breast-specific gamma imaging as an adjunct imaging modality for the diagnosis of breast cancer. Radiology. 2008; 247:651–657.
8. Zhou M, Johnson N, Gruner S, Ecklund GW, Meunier P, Bryn S, et al. Clinical utility of breast-specific gamma imaging for evaluating disease extent in the newly diagnosed breast cancer patient. Am J Surg. 2009; 197:159–163.
9. Goldsmith SJ, Parsons W, Guiberteau MJ, Stern LH, Lanzkowsky L, Weigert J. SNM practice guideline for breast scintigraphy with breast-specific gamma-cameras 1.0. J Nucl Med Technol. 2010; 38:219–224.
10. Bonhomme-Faivre L, Depraetere P, Savelli MP, Amdidouche D, Bizi E, Seiller M, et al. Charcoal suspension for tumor labelling modifies macrophage activity in mice. Life Sci. 2000; 66:817–827.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download