Abstract
Anal metastasis from colorectal cancer rarely occurs, but it severely impairs the patient's quality of life, often requiring wide resection including the anal sphincter with permanent colostomy. This lesion can be misdiagnosed as a perianal fistula or an abscess, and it can be overlooked at the time of surgery because it is not included in the routine surgical extent of low anterior resection. We report two rare cases of anal metastasis from colorectal cancer. In both cases, perianal nodules with an internal solid portion were detected on preoperative rectal magnetic resonance imaging and additional local excisions of the anal lesions were performed during the process of treatment. Anal metastasis was pathologically confirmed by histology and immunohistochemical staining.
Figures and Tables
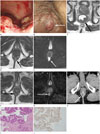 | Fig. 1An 86-year-old male presented with a complaint of intermittent hematochezia and an anal mass for two months.
A. Colonoscopy reveals an ulcerofungating tumor and the lower margin of the tumor in the rectum is 4 cm from the anal verge.
B. Another separate, palpable, fixed mass is noted at the 6 o'clock position in the anus (arrow).
C-E. Rectal MRI performed in the same patient.
C. The axial T2-weighted image of the rectum shows an ulcerofungating tumor in the posterior wall of the lower rectum (arrow), without mesorectal fat invasion.
D. The axial T2-weighted image of the rectum shows a solid nodule in the posterior side of the anal canal, intersphincteric space, 1 cm from the anal verge (arrow). The contrast enhanced T1-weighted axial image showed peripheral contrast enhancement of this nodule (not shown).
E. The diffusion weighted image (b = 800) shows diffusion restriction in the nodule (arrow).
MRI = magnetic resonance imaging
F, G. Rectal MRI performed after concurrent chemoradiotherapy for rectal cancer.
F. After concurrent chemoradiotherapy, decreased size of the primary mass (not shown) and the solid nodule posterior to the anal canal are noted on the T2-weighted image (arrow).
G. Diffusion restriction in this solid nodule on the anus is still noted on the diffusion weighted image at b = 800 (arrow). Low anterior resection and diverting loop ileostomy was performed without removal of the anal lesion.
H. Three months after the surgery, follow-up contrast enhanced CT scan shows an increase in the size of the peripheral enhancing nodule posterior to the anal canal again (arrow).
I. After local excision of the anal nodule, histopathology confirmed that the anal lesion is a moderately differentiated adenocarcinoma (hematoxylin-eosin, original magnification × 100) similar to the primary lesion.
J. Immunohistochemical staining for CDX2 in the anal lesion shows positive nuclear staining, suggesting that the lesion is a metastasis from colorectal carcinoma (immunoperoxidase, original magnification × 100).
CDX2 = caudal-related homeobox 2, CT = computed tomography
|
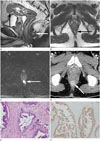 | Fig. 2A 52-year-old male presented with a complaint of change in bowel habits, and he had a previous history of a perianal abscess.
A, B. The sagittal and axial T2-weighted images of rectal MRI show a 2 cm sized indeterminate solid nodule with internal focal high signal intensity at the anal canal, in the 6 o'clock direction of the intersphincteric space (white arrow), separated from the ulcerofungating tumor at the rectosigmoid junction (black arrow) which was confirmed to be adenocarcinoma. This indeterminate nodule was regarded as a perianal abscess because of the pus-like discharge on surgical drainage and there was no evidence of malignancy at cytology.
C. This small nodule shows diffusion restriction on diffusion weighted image (b = 800) (arrow) and contrast enhancement at the periphery (not shown). After pre-operative chemotherapy and surgery including Seton procedure for the perianal abscess, low anterior resection and right hepatic lobectomy was performed. Unexpectedly, adenocarcinoma was reported in the pathologic review after Seton procedure.
D. Three months later, follow-up contrast enhanced CT scan shows an increase in the size of the enhancing nodule again at the same location of the previously treated perianal abscess (arrow).
CT = computed tomography, MRI = magnetic resonance imaging
E. After local excision of the anal nodule, histopathology confirmed that the anal lesion is a moderately differentiated adenocarcinoma (hematoxylin-eosin, original magnification × 100).
F. Immunohistochemical staining for CDX2 in the anal lesion shows positive nuclear staining, suggesting that the lesion is a metastasis from colorectal carcinoma (immunoperoxidase, original magnification × 100).
CDX2 = caudal-related homeobox 2
|
References
1. Guiss RL. The implantation of cancer cells within a fistula in ano: case report. Surgery. 1954; 36:136–139.
2. Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol. 1996; 16:619–625.
3. Takahashi H, Ikeda M, Takemasa I, Mizushima T, Yamamoto H, Sekimoto M, et al. Anal metastasis of colorectal carcinoma origin: implications for diagnosis and treatment strategy. Dis Colon Rectum. 2011; 54:472–481.
4. Ishiyama S, Inoue S, Kobayashi K, Sano Y, Kushida N, Yamazaki Y, et al. Implantation of rectal cancer in an anal fistula: report of a case. Surg Today. 2006; 36:747–749.
5. Benjelloun el B, Aitalalim S, Chbani L, Mellouki I, Mazaz K, Aittaleb K. Rectosigmoid adenocarcinoma revealed by metastatic anal fistula. The visible part of the iceberg: a report of two cases with literature review. World J Surg Oncol. 2012; 10:209.
6. Rosser C. The aetiology of anal cancer. Am J Surg. 1931; 11:328–333.
7. Ramalingam P, Hart WR, Goldblum JR. Cytokeratin subset immunostaining in rectal adenocarcinoma and normal anal glands. Arch Pathol Lab Med. 2001; 125:1074–1077.
8. Lisovsky M, Patel K, Cymes K, Chase D, Bhuiya T, Morgenstern N. Immunophenotypic characterization of anal gland carcinoma: loss of p63 and cytokeratin 5/6. Arch Pathol Lab Med. 2007; 131:1304–1311.
9. Morris J, Spencer JA, Ambrose NS. MR imaging classification of perianal fistulas and its implications for patient management. Radiographics. 2000; 20:623–635. discussion 635-637.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download