Abstract
Hepatoid adenocarcinoma is a rare extrahepatic tumor defined as having a morphologic and immunohistochemical similarity to hepatocellular carcinoma. In this case report, we describe a patient with hepatoid adenocarcinoma of the jejunum with multiple liver metastases that developed in the absence of risk factors. We describe the radiologic findings including those of dynamic computed tomography and small bowel follow-through. To the best of our knowledge, only two cases of hepatoid adenocarcinoma of the small bowel have been reported in patients with inflammatory bowel disease.
Hepatoid adenocarcinoma is a special type of extrahepatic adenocarcinoma that mimics hepatocellular carcinoma morphologically and immunohistochemically, and also shares the alpha-fetoprotein (AFP) producing feature (1). Because of the poor prognosis of this histologic tumor type, accurate diagnosis of hepatoid adenocarcinoma is important for developing the treatment plan. Hepatoid adenocarcinomas have been reported in various organs, including esophagus, stomach, colon, pancreas, gall bladder, lung, urinary bladder, ovaries and uterus (23456). Among them, imaging findings of hepatoid adenocarcinomas of the stomach have been reported in a large series to date (2).
With respect to hepatoid adenocarcinoma of the small bowel, only two cases have been reported in patients with long-standing Crohn's disease (67). In addition, to the best of our knowledge, there is no report describing the radiologic features of hepatoid adenocarcinoma of the small bowel in a patient who did not have inflammatory bowel disease or other risk factors. We describe a case of hepatoid adenocarcinoma of the jejunum with hepatic metastases that developed in a patient who did not have any risk factors for small bowel cancer and discuss the imaging features in terms of dynamic CT and small bowel followthrough.
A 48-year-old man was referred to our hospital for evaluation of a huge hepatic tumor that was detected during abdominal sonographic examination at an outside hospital.
On physical examination, the patient appeared to be acutely ill with a blood pressure of 135/80 mm Hg, heart rate of 75 beats/min and temperature of 37℃. He had no remarkable previous medical history including viral hepatitis or chronic alcoholism. Laboratory tests revealed a decreased hemoglobin level of 6.8 g/dL (normal range, 13.0–17.0 g/dL) and a slightly elevated alkaline phosphatase level of 275 IU/L (normal range, 40–250 IU/L), but normal levels of serum liver enzymes (an aspartate aminotransferase level of 31 IU/L; an alanine aminotransferase level of 30 IU/L) and a total bilirubin level of 0.62 mg/dL. The levels of serum AFP (571 ng/mL; normal range, < 9.6 ng/mL) and CA19-9 (carbohydrate antigen 324 U/mL; normal range, < 37 U/mL) were markedly elevated. The polyclonal carcinoembryonic antigen (CEA 7.0 ng/mL) and protein levels induced by vitamin K absence or antagonist-II (99 mAU/mL) were slightly elevated. His serum was positive for hepatitis B surface antibody, but negative for hepatitis B surface antigen and anti-HCV.
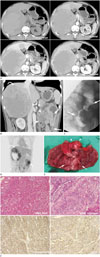
The radiograph of the abdomen showed no detectable abnormality. We performed dynamic CT to evaluate the liver mass and CT images revealed multiple heterogeneously and peripherally enhanced masses in both lobes of the liver. These masses showed persistent and more enhancement in the portal and delayed phases than in the arterial phase (Fig. 1A). The largest tumor was a 14 × 12 cm-sized mass, comprised of a central high density (56 Hounsfield units) area on the pre-contrast scan without gross contrast enhancement, suggesting hemorrhage or necrosis. Cirrhotic change in the liver was not observed. Also, CT scans showed 6.5 cm-long eccentric irregular enhancing wall thickening and perilesional infiltration in the proximal jejunum. Several adjacent lymph nodes were enlarged (up to 4 cm) with some internal low attenuation (Fig. 1B). Double contrast small bowel follow-through was performed to evaluate the small bowel lesion, and it showed a short, circumferentially narrowed segment with irregular mucosal disruption and ulcer in the proximal jejunum (Fig. 1C). We suggested small bowel adenocarcinoma of the jejunum, metastatic regional lymph node enlargement, and multiple hepatic metastases. On the positron emission tomography image, multiple focal strong fluorodeoxyglucose (FDG) uptakes, a 10 cm-sized well-defined mass in the liver, and focal small bowel wall thickening with strong focal FDG uptake (standardized uptake value = 6.25) were noted (Fig. 1D).
The surgeon performed segmental resection of the proximal jejunum and biopsy of the liver mass to confirm the tumor origin, to determine the treatment option, and to relieve the symptoms of small bowel obstruction. The gross pathology of the proximal jejunum showed an irregular transluminal mass with infiltration and enlarged regional lymph nodes (Fig. 1E). Hematoxylin and eosin staining of the tumor cells from the proximal jejunum revealed clear cell cytoplasm which is a more prevalent finding in hepatoid adenocarcinoma than in adenocarcinoma. Immunohistochemical staining of the tumor cells from the jejunum with hepatocyte specific antigen and polyclonal CEA antibody showed diffuse positivity (Fig. 1F). Biopsy of the liver mass confirmed the diagnosis of metastatic carcinoma, clinically from the small intestine.
Hepatoid adenocarcinoma is a distinctive type of extrahepatic adenocarcinoma that mimics hepatocellular carcinoma morphologically and immunohistochemically, and it is characterized by a poor prognosis (6). These tumors have been most frequently reported in the stomach, but sporadic cases have also been described in other parts of the gastrointestinal tract and extra gastrointestinal organs such as pancreas, gall bladder, ovary, lung and testis. Only two cases of hepatoid adenocarcinomas of the small bowel have been reported in patients with inflammatory bowel disease (67). Our case can be the third case of hepatoid adenocarcinoma of the small bowel, and to the best of our knowledge, it is the first case to occur in a patient without an underlying risk factor such as inflammatory bowel disease. Hepatocellular carcinoma and hepatoid adenocarcinoma share numerous clinicopathological features like an elevated level of serum AFP, hepatoid morphology and immunoreactivity for AFP and CEA (1). In our case, immunohistochemical staining showed positivity for AFP and CEA. Furthermore, in our patient, the serum AFP level was elevated as in patients with many other hepatoid adenocarcinomas. But all cases of hepatoid adenocarcinomas have not been reported with an elevated serum AFP level. Some cases of hepatoid adenocarcinoma of the stomach did not show elevated serum AFP and they were associated with absence of hepatic metastases. Hence, in one report, the authors suggested that the normal level of serum AFP in patients with hepatoid adenocarcinoma could be correlated with a better prognosis (8). One review of hepatoid adenocarcinoma reported that the median survival was 11 months and that poor prognosis of hepatoid adenocarcinoma was correlated with extensive vascular and lymphatic invasion and hepatic metastases (6).
In our case, double contrast small bowel follow-through showed a short segmental mass with luminal narrowing and shouldering in the proximal jejunum and these findings were relatively compatible with findings of adenocarcinoma of the small bowel. The post contrast enhanced CT scan showed an irregular enhancing mass in the proximal jejunum, enlarged regional lymph nodes and multiple huge peripherally enhancing hepatic masses. In a large series of hepatoid adenocarcinoma of the stomach, eccentric wall thickening, heterogeneous enhancement, adjacent organ invasion, regional lymphadenopathy, distant metastasis, and venous tumor thrombosis could be the radiologic findings of hepatoid adenocarcinoma (25). In our case, similar imaging findings were noted except for adjacent organ invasion and venous thrombosis. We thought that the imaging finding of hepatoid adenocarcinoma of the small bowel could be similar to those of tumor of the stomach. With respect to liver metastases, dynamic CT showed a dominant bulky mass similar to that in previous studies (25) along with several small masses. Instead of the arterial wash-in and delayed wash-out pattern, delayed peripheral enhancement was noted just like in usual hepatic metastases. Considering these imaging findings, our case of hepatoid adenocarcinoma is more prone to metastasis than hepatocellular carcinoma (910). Like in other articles (2345), our case of hepatoid adenocarcinoma also showed aggressive behavior due to extensive hepatic metastases and metastatic lymphadenopathy.
In conclusion, we report a case of hepatoid adenocarcinoma of the small bowel that developed in patient who did not have any risk factors and describe the imaging findings of the mass and hepatic metastases on small bowel follow-through and multiphase CT scan. The radiologist should consider the possibility of hepatoid adenocarcinoma in the differential diagnosis of small bowel cancer if there are huge liver metastases with elevated serum AFP.
Figures and Tables
Fig. 1
A 48-year-old man with a liver mass detected on ultrasonography.
A. Multiphase contrast enhanced CT images show multiple, large liver masses with heterogeneous and delayed peripheral enhancement (pre contrast CT; 35 HU, arterial phase; 69 HU, portal phase; 79 HU, and delayed phase; 69 HU, center of the mass 56 HU in all phases). HU = Hounsfield units
B. Coronal reformatted post contrast enhanced CT scan shows an irregular mass in the proximal jejunum (arrows), enlarged regional lymph nodes (arrowheads), and a huge liver mass (curved arrows).
C. Small bowel follow-through shows a focal luminal mass with shouldering and irregular mucosal destruction (arrows).
D. PET shows multiple focal strong FDG uptakes in both lobes with a large FDG defect lesion (curved arrow) in the right lobe of the liver and focal and marked wall thickening of the small bowel (arrow) with strong FDG uptake.
E. Gross specimen shows a huge, ill-defined, ulcerated mass (arrow), measuring about 8 × 10 cm and multiple enlarged lymph node (arrowheads) with a necrotic surface. FDG = fluorodeoxyglucose, PET = positron emission tomography
F. Histologic specimen shows diffuse positivity for antihepatocyte specific antigen and polyclonal carcinoembryonic antigen (CEA) antibody. H&E = hematoxylin and eosin
References
1. Supriatna Y, Kishimoto T, Uno T, Nagai Y, Ishikura H. Evidence for hepatocellular differentiation in alpha-fetoprotein-negative gastric adenocarcinoma with hepatoid morphology: a study with in situ hybridisation for albumin mRNA. Pathology. 2005; 37:211–215.
2. Lee MW, Lee JY, Kim YJ, Park EA, Choi JY, Kim SH, et al. Gastric hepatoid adenocarcinoma: CT findings. Abdom Imaging. 2007; 32:293–298.
3. Kato K, Suzuka K, Osaki T, Itami M, Tanaka N. Primary hepatoid adenocarcinoma of the uterine cervix. Int J Gynecol Cancer. 2007; 17:1150–1154.
4. Lopez-Beltran A, Luque RJ, Quintero A, Requena MJ, Montironi R. Hepatoid adenocarcinoma of the urinary bladder. Virchows Arch. 2003; 442:381–387.
5. Wu Z, Upadhyaya M, Zhu H, Qiao Z, Chen K, Miao F. Hepatoid adenocarcinoma: computed tomographic imaging findings with histopathologic correlation in 6 cases. J Comput Assist Tomogr. 2007; 31:846–852.
6. Kochar K, Gawart M, Atkinson J, Hyser M. Hepatoid adenocarcinoma of small intestine complicating Crohn's disease: second reported case. J Gastrointest Cancer. 2013; 44:357–361.
7. Liu Q, Bannan M, Melamed J, Witkin GB, Nonaka D. Two cases of hepatoid adenocarcinoma of the intestine in association with inflammatory bowel disease. Histopathology. 2007; 51:123–125.
8. Qu BG, Bi WM, Qu BT, Qu T, Han XH, Wang H, et al. PRISMA-compliant article: clinical characteristics and factors influencing prognosis of patients with hepatoid adenocarcinoma of the stomach in China. Medicine (Baltimore). 2016; 95:e3399.
9. Terracciano LM, Glatz K, Mhawech P, Vasei M, Lehmann FS, Vecchione R, et al. Hepatoid adenocarcinoma with liver metastasis mimicking hepatocellular carcinoma: an immunohistochemical and molecular study of eight cases. Am J Surg Pathol. 2003; 27:1302–1312.
10. Jo JM, Kim JW, Heo SH, Shin SS, Jeong YY, Hur YH. Hepatic metastases from hepatoid adenocarcinoma of stomach mimicking hepatocellular carcinoma. Clin Mol Hepatol. 2012; 18:420–423.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download