Abstract
Purpose
To describe imaging features of infratentorial desmoplastic infantile or non-infantile tumors (DIT/DNIT).
Materials and Methods
Four cases with infratentorial DIT/DNIT from our hospital and 5 cases from literature review were analyzed. Clinical data and MR imaging features were evaluated including location, size, shape, margin, composition, dural attachment, perilesional edema, and metastasis or multiplicity.
Results
The mean age was 9.2 years (range, 1–18 years). Most of the patients presented with headache or vomiting (4/9, 44.4%) and had no underlying disease (8/9, 88.9%). The major pathologic subtype was astrocytoma (6/9, 66.7%). On MR, majority of the tumors involved cerebellum and/or spinal cord (8/9, 88.9%) and the mean size of the tumors was 4.2 cm (range, 3.2–5 cm). The tumors were mainly solid (4/9, 44.4%) or mixed (4/9, 44.4%) in composition with lobulated shape (7/9, 77.8%) and well-defined margin (7/9, 77.8%). Two cases (2/7, 28.6%) showed dural attachment and all the cases had no or minimal perilesional edema (100%). Metastasis or multiplicity was frequently seen in 44.4% (4/9).
Desmoplastic infantile or non-infantile tumors (DIT/DNIT) were first described in 1987 (1) and are rare supratentorial brain tumors (2). These tumors involve a desmoplastic reaction with a dura mater adherent portion. The cellular areas of the tumor are characterized by neural cells of variable degrees of maturation (3), which differentiate desmoplastic infantile ganglioglioma and astrocytoma. They are classified together in the World Health Organization classification as grade I (benign) (4).
The reported typical imaging features of DIT/DNIT are supratentorial masses with a large cystic portion, small peripheral solid portion with contrast enhancement, predilection for frontal and parietal lobes, and meningeal enhancement suggesting dural attachment (56). However, since initially reported, more tumor cases with atypical imaging findings have been reported including tumors with less cystic and more solid portions, location outside the supratentorium, and no dural attachment (78910111213141516). To the best of our knowledge, no study has systematically reviewed MR images of infratentorial DIT/DNIT including both posterior fossa and spinal cord. Therefore, the purpose of this study was to review the MR imaging features of infratentorial DIT/DNIT from our hospital and from literature search.
The Institutional Review Board of our hospital approved this investigation, and written informed consent was waived. We retrospectively reviewed pathologically-confirmed DIT or DNIT cases in our hospital between 2005 and 2014. There were 5 cases of DIT identified in our database. One case was supratentorial DIT/DNIT and excluded. Ultimately, 4 cases from our hospital were enrolled in the study.
A comprehensive literature search using PubMed, a database created by the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/), was conducted in April 2014. The key words were "desmoplastic infantile," "desmoplastic non infantile," and "desmoplastic non-infantile" and 112, 19, and 4 reports were retrieved using these key words, respectively. Among a total of 135 reports, 12 reports were duplicates and removed. Additionally, 69 reports were excluded for the following reasons: 30 reports had insufficient information of tumor location; 29 reports were on an unrelated topic, such as review or epidemiology studies; 6 reports were written in a language other than English; and 4 reports did not have a full-text article available. Finally, 54 reports with 75 eligible cases were reviewed. Regarding the location, the tumor was classified as infratentorial if the main tumor was outside the intracranial supratentorial area, including the posterior fossa and spinal cord. Cases with multiple tumors in both supratentorial and infratentorial locations but without dominant tumor in supratentorial area were classified as infratentorial. Finally, among the 54 reports, 5 reports with 5 cases of infratentorial DIT/DNIT were reviewed for this study.
We collected data on clinical features, including gender and age. If possible, presenting symptom, underlying disease, treatment methods, follow-up duration, recurrence, and survival were recorded. Pathologic results and subtypes (ganglioglioma or astrocytoma) were also reviewed with the cellularity of the tumors by a pathologist.
MR images were obtained in our institution by use of a 3.0-T unit equipped with a head coil (Achieva; Philips Medical Systems, Amsterdam, the Netherlands). The study included T1-weighted image [T1-WI, repetition time (TR) = 2000 or 2300 ms, echo time (TE) = 10 ms, matrix = 190–203 × 256, flip angle = 90°, field of view = 230 × 230 mm, number of acquisition = 1], T2-weighted image (T2-WI, TR = 3000 or 6450 ms, TE = 80 ms, matrix = 319 × 400 or 329 × 448, flip angle = 90°, field of view = 230 × 230 mm, number of acquisition = 1), and T1-WI with gadolinium enhancement.
For MR image analysis, 2 radiologists with 4 and 10 years of experience, respectively, reviewed the MR images with a consensus. When evaluating the subjects from the literature search, both figures in the articles and the description of the MR images were reviewed and collected. MR image characteristics included the following: location, size, shape (round, oval, lobulated, or irregular), margin (well-defined or ill-defined), composition (mixed solid and cystic, mainly solid, or mainly cystic), internal content (calcification, hemorrhage, or fat), dural attachment (absent or present), perilesional edema (no/minimal or massive), skull change, associated brain anomaly, and metastasis or multiplicity (absent or present) at the time of diagnosis. Regarding size, the largest tumor diameter was measured or recorded in a case with multiple lesions. Regarding composition, the tumor was classified as 'mainly solid' if the tumor was > 90% solid and as 'mainly cystic' if there was no enhancing solid portion. For the evaluation of internal content, CT images were also reviewed, if available. Metastasis or multiplicity was classified as 'present' if there were multiple lesions or leptomeningeal dissemination at the time of diagnosis.
Signal intensities on MR imaging were evaluated. The signal intensities of the solid portion of the tumor were described on T1-WI, T2-WI, and T1-WI with gadolinium enhancement. Additionally, the imaging findings were also described for other sequences such as diffusion-weighted image or MR spectroscopy.
There were 5 male and 4 female patients. The mean age was 9.2 ± 4.9 years (range: 1–18 years). Most of the patients were > 2 years of age (8/9, 88.9%). Headache or vomiting was the most common presenting symptom (4/9, 44.4%), followed by neck stiffness (2/9, 22.2%), ataxia or bilateral lower limb weakness (2/9, 22.2%), and increase in head circumference (1/9, 11.1%). None of the patients had underlying disease except for 1 patient who had clavicular arteriovenous malformation. All patients underwent tumor removal for the treatment; 1 underwent neoadjuvant chemotherapy and 4 underwent adjuvant chemo- or radiotherapy. Follow-up was specified in 8 cases, except for 1 case from Santhosh et al. (11), with a mean duration of 30.6 ± 12.7 months (range, 7–48 months). There was no reported case of recurrence or death during the follow-up period.
The most common pathologic subtypes were astrocytoma (6/9, 66.7%). The solid portion of the tumors from our hospital showed similar cellularity compared to pilocytic astrocytoma and relatively lower cellularity compared to medulloblastoma.
MR imaging features of the 9 cases of infratentorial DIT/DNIT were summarized in Table 1. Seven cases were strictly of infratentorial locations and the other 2 cases (Case 5 and 8) were of both supratentorial and infratentorial locations. The majority of the cases demonstrated cerebellar (4 cases) and/or spinal cord (4 cases) involvement. All the spinal cord lesions showed intramedullary location.
The mean tumor size was 4.2 cm (range: 3.5–5 cm) in the 6 specified cases. Most of the tumors showed lobulated shape (7/9, 77.8%) and the other 2 showed round or oval shape (Case 6 and 8). None of the tumors had irregular shape. The majority of the cases demonstrated well-defined margin (7/9, 77.8%) and 2 (Cases 2 and 4) showed ill-defined margin. Regarding the tumor composition, 4 cases (4/9, 44.4%) were mainly solid (Fig. 1) and 4 cases (4/9, 44.4%) were mixed (Table 1). The solid portion of the tumors showed iso to low signal intensities on T1-WI and iso to high signal intensities on T2-WI compared to gray matter. Two of 9 cases (22.2%) had gross calcification and 2 cases had microscopic level calcification. One case (Case 2) had a fat component inside the tumor (Fig. 2) and there was no case with hemorrhage. All the solid portions of the tumors showed contrast enhancement. Only 1 case (Case 3) was mainly cystic in composition and demonstrated multiple small cystic lesions at the time of diagnosis (Fig. 3).
Dural attachment was present in 2 cases (Case 3 and 8) among 7 specified cases (28.6%). All cases had no or minimal perilesional edema (100%). Associated skull change or brain anomaly was absent in all cases. In addition, combined hydrocephalus (5/9, 55.6%) or syringomyelia (1/9, 11.1%) were presented. Four cases (4/9, 44.4%) presented with metastasis or multiplicity at the time of diagnosis; 3 cases (Case 2, 3, and 8) showed multiple cystic lesions and 1 (Case 9) showed leptomeningeal dissemination.
Diffusion-weighted images were included in 3 cases (Case 2, 3, and 8) and only the mainly solid tumor (Case 2) showed diffusion restriction. MR spectroscopy was performed in 1 case (Case 8) and the results showed as an elevated choline peak and a reduced N-acetylaspartate peak.
In this study, we reviewed cases of infratentorial DIT/DNIT. The most common pathologic subtype of these tumors was astrocytoma and the most common imaging features were mainly solid or mixed in composition with frequent metastasis or multiplicity at the time of diagnosis.
There is no standard age dividing DIT and DNIT. Since the term 'infant' can be used for children from birth to 2 years old, we divided cases into age groups at this age; most of the patients (8/9) with infratentorial DIT/DNIT were older than 2 years of age in our study.
The typical composition of supratentorial DIT/DNIT on MR imaging is reportedly mixed solid and cystic with the solid portion showing contrast enhancement (6). Studies with serial comparisons of the DIT/DNIT have suggested that a primary solid tumor could progress to cystic lesions due to cerebrospinal fluid entrapment (1117). In our study, infratentorial tumors also showed similar composition. Most tumors (8/9, 88.9%) showed mainly solid or mixed in composition.
Previously, dural attachment showing enhancement along the dura on MR images was considered a characteristic finding of supratentorial DIT/DNIT (618). However, a review on DIT/DNIT revealed the absence of dural attachment in 41% of cases (18). In our study, dural attachment was present only in 2 cases (28.6%). Therefore, absent dural attachment cannot exclude the possibility of this rare tumor, especially when the tumor originates in an infratentorial site.
Although DIT/DNIT are generally known as benign tumors, there were cases with metastasis or multiplicity at the time of diagnosis (4/9, 44.4%). Three of 4 cases with metastasis or multiplicity in the infratentorial DIT/DNIT showed multiple cystic lesions. Santhosh et al. (11) reported a case of multifocal desmoplastic astrocytoma (Case 8 in our study) and discussed the possibility of both metachronous tumors and metastasis. We also did not differentiate these two in cases with multiple lesions in our study. However, a previous study reported only 1 case with multiple locations of tumor among 88 cases (1/88, 1.1%) of general DIT/DNIT (18). The high proportion of metastasis or multiplicity in our study could be due to a selection bias. Moreover, no cases showed recurrence or death during the follow up in our study. Further study to determine differences in frequency or characteristic finding of metastasis or multiplicity between supratentorial and infratentorial tumors is needed with a larger number of cases.
Infratentorial DIT/DNIT should to be differentiated from pilocytic astrocytoma and medulloblastoma. Pilocytic astrocytomas most often have a large cystic portion with a solid mural nodule (19). Most of the medulloblastomas show iso to low signal intensities on T2-WI (19). However, most of the infratentorial cases in our report were mainly solid or mixed in composition and had signal intensities of the solid portion that were iso to high on T2-WI. This signal on T2-WI can be interpreted by relatively lower cellularity of the tumor compared to medulloblastoma. DIT/DNIT in a spinal cord should be differentiated from astrocytoma and ependymoma. As opposed to the astrocytomas, which are eccentrically located and enhance less intensely (20), all the lesions within the spinal cord in our study showed intramedullary central location and good enhancement. In our study, we observed no peripheral hemosiderin rim in the spinal cord involving DIT/DNIT, as compared to ependymoma (21). However; distinguishing between the spinal cord DIT/DNIT and the two common spinal cord tumors may be difficult due to absence of specific features, as shown in our study.
The small number of cases is the major limitation of this study. The limited number might have exaggerated our findings regarding tumor characteristics. In addition, there is an issue of heterogeneous location of the cases as 2 of 9 cases involved both supratentorial and infratentorial sites. However, the majority of cases involved the cerebellum and/or spinal cord. Further study with a large number of cases and comparative study including both supratentorial and infratentorial DIT/DNIT is needed.
In conclusion, we demonstrated the clinical and imaging findings of the rare condition of infratentorial DIT/DNIT. Infratentorial tumors occurred in older children with the majority as astrocytoma. On imaging, infratentorial tumors were mostly solid or mixed in composition with frequent metastasis or multiplicity.
Figures and Tables
Fig. 1
Case 1. A six-year-old boy with desmoplastic non-infantile astrocytoma.
A mass involving the medulla oblongata and upper cervical spinal cord demonstrates mainly solid composition with isointense to parenchyma on T1-weighted image (A), high signal intensity on T2-weighted image (B, C), and heterogeneous enhancement in the solid portion on contrast enhancement study (D).
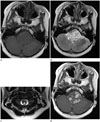
Fig. 2
Case 2. A nine-year-old boy with desmoplastic non-infantile astrocytoma.
A mass involving the cerebellum and midbrain shows high signal intensity areas of fat on T1-weighted image (A), solid portion with multiple small cystic lesions on T2-weighted image (B), and heterogeneous enhancement in the solid portion on contrast enhancement study (C).
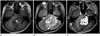
Fig. 3
Case 3. A nine-year-old girl with desmoplastic non-infantile astrocytoma.
A mass in the right cerebellum demonstrates entirely cystic on T2-weighted image (A), internal septation and dural attachment (arrow) on contrast enhancement T1-weighted image (B), and multiple small cystic lesions in the cerebellum (C).
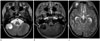
Table 1
Imaging Features of Nine Cases of Desmoplastic Infantile and Non-Infantile Tumors Located at Infratentorial Sites

References
1. VandenBerg SR, May EE, Rubinstein LJ, Herman MM, Perentes E, Vinores SA, et al. Desmoplastic supratentorial neuroepithelial tumors of infancy with divergent differentiation potential ("desmoplastic infantile gangliogliomas"). Report on 11 cases of a distinctive embryonal tumor with favorable prognosis. J Neurosurg. 1987; 66:58–71.
2. Rout P, Santosh V, Mahadevan A, Kolluri VR, Yasha TC, Shankar SK. Desmoplastic infantile ganglioglioma--clinicopathological and immunohistochemical study of four cases. Childs Nerv Syst. 2002; 18:463–467.
3. Ng TH, Fung CF, Ma LT. The pathological spectrum of desmoplastic infantile gangliogliomas. Histopathology. 1990; 16:235–241.
4. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114:97–109.
5. Tenreiro-Picon OR, Kamath SV, Knorr JR, Ragland RL, Smith TW, Lau KY. Desmoplastic infantile ganglioglioma: CT and MRI features. Pediatr Radiol. 1995; 25:540–543.
6. Trehan G, Bruge H, Vinchon M, Khalil C, Ruchoux MM, Dhellemmes P, et al. MR imaging in the diagnosis of desmoplastic infantile tumor: retrospective study of six cases. AJNR Am J Neuroradiol. 2004; 25:1028–1033.
7. De Munnynck K, Van Gool S, Van Calenbergh F, Demaerel P, Uyttebroeck A, Buyse G, et al. Desmoplastic infantile ganglioglioma: a potentially malignant tumor? Am J Surg Pathol. 2002; 26:1515–1522.
8. Milanaccio C, Nozza P, Ravegnani M, Rossi A, Raso A, Gambini C. Cervico-medullary desmoplastic infantile ganglioglioma: an unusual case with diffuse leptomeningeal dissemination at diagnosis. Pediatr Blood Cancer. 2005; 45:986–990.
9. Darwish B, Arbuckle S, Kellie S, Besser M, Chaseling R. Desmoplastic infantile ganglioglioma/astrocytoma with cerebrospinal metastasis. J Clin Neurosci. 2007; 14:498–501.
10. Lönnrot K, Terho M, Kähärä V, Haapasalo H, Helén P. Desmoplastic infantile ganglioglioma: novel aspects in clinical presentation and genetics. Surg Neurol. 2007; 68:304–308. discussion 308.
11. Santhosh K, Kesavadas C, Radhakrishnan VV, Abraham M, Gupta AK. Multifocal desmoplastic noninfantile astrocytoma. J Neuroradiol. 2008; 35:286–291.
12. Rasalkar DD, Paunipagar BK, Ng A. Primary spinal cord desmoplastic astrocytoma in an adolescent: a rare tumour at rare site and rare age. Hong Kong Med J. 2012; 18:253–255.
13. Gundamaneni SK, Ganesh CV, Mahadevan A, Madhugiri VS, Sasidharan GM, Kumar VR. Non-infantile desmoplastic cerebellar ganglioglioma in a patient with multiple congenital anomalies: a rare association. Pediatr Neurosurg. 2013; 49:105–109.
14. Taranath A, Lam A, Wong CK. Desmoplastic infantile ganglioglioma: a questionably benign tumour. Australas Radiol. 2005; 49:433–437.
15. Setty SN, Miller DC, Camras L, Charbel F, Schmidt ML. Desmoplastic infantile astrocytoma with metastases at presentation. Mod Pathol. 1997; 10:945–951.
16. Karabagli P, Karabagli H, Kose D, Kocak N, Etus V, Koksal Y. Desmoplastic non-infantile astrocytic tumor with BRAF V600E mutation. Brain Tumor Pathol. 2014; 31:282–288.
17. Taguchi Y, Sakurai T, Takamori I, Sekino H, Tadokoro M. Desmoplastic infantile ganglioglioma with extraparenchymatous cyst--case report. Neurol Med Chir (Tokyo). 1993; 33:177–180.
18. Gelabert-Gonzalez M, Serramito-García R, Arcos-Algaba A. Desmoplastic infantile and non-infantile ganglioglioma. Review of the literature. Neurosurg Rev. 2010; 34:151–158.
19. Tortori-Donati P, Rossi A, Biancheri R, Garrè ML, Cama A. Brain tumors. In : Tortori-Donati P, Rossi A, Biancheri R, Garrè ML, Cama A, editors. Pediatric neuroradiology. Berlin: Springer;2005. p. 329–436.
20. Houten JK, Weiner HL. Pediatric intramedullary spinal cord tumors: special considerations. J Neurooncol. 2000; 47:225–230.
21. Nemoto Y, Inoue Y, Tashiro T, Mochizuki K, Oda J, Kogame S, et al. Intramedullary spinal cord tumors: significance of associated hemorrhage at MR imaging. Radiology. 1992; 182:793–796.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download