Abstract
Lung cancer is one of the most commonly diagnosed cancers, and the lungs are a common site of metastasis from extrathoracic malignancies. Surgical resection is the gold standard treatment for lung malignancies. However, some of the patients are poor surgical candidates due to various reasons. Currently, image-guided ablation is used as one of the lung cancer treatment modalities. Cryoablation has been adapted as one of the treatments of lung tumors and a growing body of literature has shown that it is a safe and effective option. We report a case of successful cryoablation for a metastatic lesion from surgically resected primary lung cancer.
The lungs are a common site for malignancy, either primary or metastases. Surgical resection is the treatment of choice for lung malignancies. However, some patients are not good candidates for lung resection due to various reasons, including inability to meet the criteria for lung operation, patient's poor general conditions such as cardiovascular disorders, impaired pulmonary function, or even patients' refusal for surgery.
For cases of inoperable focal malignancies, image-guided percutaneous cryoablation is currently evolving as a minimally invasive and potentially effective treatment option. Unlike heat-based therapies such as microwave and radiofrequency ablation, cryoablation is based on the therapeutic application of extreme cold to living tissue in order to cause local destruction. Advantages of cryoablation include good monitoring by CT during the procedure resulting in minimizing the risk of injury to nearby structures, sparing collagen containing structures and causing less pain than radiofrequecy ablation (RFA). In this report, we present a case of successful cryoablation which was performed for a small metastatic lesion from surgically resected primary lung cancer.
A 76-year-old male patient underwent left upper lobectomy for lung adenocarcinoma in July 2010. The pathologic stage was pT3 (separate nodule in the same lobe) N0M0, stage IIB. He had received adjuvant chemotherapy. Three years after left upper lobectomy, a metastatic nodule was found in the right upper lobe and wedge resection was performed for the lesion. Then, he underwent radiation therapy. Fifteen months after metastasectomy of the nodule in the right upper lobe, an approximately 1.5 cm sized subpleural nodule was found on the follow up CT scan (Fig. 1). CT-guided percutaneous needle biopsy was performed for the lesion and it was proved to be another recurred nodule of adenocarcinoma. Through a multidisciplinary approach, the patient was referred to our department for cryoablation of the recurred malignant lesion. Considering that he had already received chemotherapy and radiation therapy and that he also had impaired pulmonary function, we came to the conclusion that one of the ablation therapies would be the best treatment option. The reason for choosing cryoablation among the possible ablation therapies was the several advantages that it offers, including that it is minimally invasive, easy to apply, real-time monitoring is feasible, less painful, and spares the adjacent collagen containing structures.
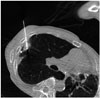
Percutaneous cryoablation was performed under CT guidance, in the following manner: first, a limited non contrast low-dose CT scan (80 kV, 30 mAs, 1.2 mm slice) was performed to localize the target lesion. The patient was placed in a supine position with slight right anterior oblique rotation and draped in the usual fashion. Lidocaine solution (1%) was infiltrated subcutaneously into the soft tissue adjacent to the puncture site. Under the guidance by CT fluoroscopy (Siemens Somatom Definition Flash, Forchheim, Germany), the cryoprobe was inserted into the target nodule in the right upper lobe (Fig. 2). The cryotherapy equipment that we used consisted of an argon: helium gas-based system (SeedNet Gold™ system, Galil Medical Ltd., Yokneam, Israel). Three cycles of freezing and thawing were performed. In the first cycle, 3-minute freezing followed by 5-minute thawing, in the second cycle, 7-minute freezing and 10-minute thawing, and in the final cycle, 10-minute freezing and 3-minute active thawing were performed. After removing the probe, the incision site was covered as usual with a proper ointment dressing. During the procedure, the patient had a tolerable experience and there was no evidence of any immediate procedure-related complications. After ablation, the patient was monitored by checking his vital signs every 2 hours during the half day. On the next day of ablation, the patient was discharged without any specific complaints. Follow up chest radiography 4 hours after the procedure and CT scan 1 week after the ablation showed no evidence of procedure-related complications such as pneumothorax, hemothorax or pulmonary hemorrhage. Technical success is commonly defined as whether the treatment was performed according to the protocol with complete coverage of the tumor. By evaluating the CT scan images acquired during the procedure, we ensured technical success and complete coverage was obtained.
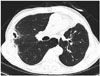
To evaluate the effectiveness of the technique, we compared the CT scan performed before cryoablation with CT scans performed one, three, and five months after the procedure. On the follow-up CT scans, the treated lesion showed nearby necrosis and cavitation, suggesting successful ablation (Fig. 3).
Image-guided tumor ablation by thermal or non-thermal sources has received increased attention for managing focal malignancies, although the primary management options for lung cancers traditionally depend on the surgical treatment. Due to the increase in the number of screening studies for lung cancer, we can detect small nodules and early stage lung cancers, and nowadays the trend of organ conserving treatment is becoming popular. Hence, the local and less invasive treatment modalities have a high demand.
Cryoablation is usually performed for tumors less than 3 cm in size. In tumors greater than 3 cm in size, there is a higher chance of recurrence than in smaller tumors. Cryoablation is known to be a safer modality for tumors close to large vascular structures as well as tumors located near the chest wall. Percutaneous cryoablation enables preservation of the collagenous architecture of the bronchi and the vascular structures, and this advantage allows performing cryoablation for tumors located immediately adjacent to the central bronchi. This may be the main advantage of cryoablation, compared to heat-based treatments. Also, it causes less pain than radiofrequency ablation; hence, it is recommended for patients with less tolerability. Since about 2–3 years, cryoablation for malignant tumors has been covered by medical insurance in Korea.
Current cryoablation systems use the Joule-Thomson effect to create freeze and thaw cycles. The Joule-Thomson effect describes the change in temperature of a gas resulting from expansion or compression of that gas. Gas expansion occurs in a small chamber inside the distal end of the cryoprobe to create the necessary heat sink during freeze cycles and heat source during thaw cycles (1).
During rapid tissue cooling, water is entrapped within the cell membrane, resulting in intracellular ice formation, and can cause recrystallization and extension of the ice within the intracellular matrix. These changes induce enzyme dysfunction and cell mem-brane dysfunction. In tumors with a slowing cooling process, there is ice formation in the extracellular matrix, resulting in osmosis of water out of the cell. Also, there are many collateral effects of extreme cold, occurring along the blood vessel wall. In blood vessels, direct contact with rapid cooling process, there are similar direct effects. These indirect effects in concert with direct effects result in coagulation necrosis. To achieve an effective cryoablation tissue injury, excellent monitoring of the process, fast cooling to a lethal temperature, slow thawing and repetition of freeze-thaw cycle are the critical factors.
Possible complications after cryoablation range from pneumothorax, hemoptysis, pleural effusion, fever, hypertension, subcutaneous emphysema, skin injury, infection, to nerve injuries. The most common complication encountered with cryoablation is pneumothorax, occurring approximately 12–62% of the patients after cryoablation (23456). The reported rates of hemoptysis after cryoablation range from 0% to 62% (23456).
CT is a useful modality to evaluate the treated lesion and to monitor the local tumor progression. There is considerable variation in how an ablated zone around the target tumor responds after treatment and progression occurs during follow-up. Immediate post-ablation CT scans typically show a definite low attenuation area surrounding the ablated foci, suggesting tumor necrosis, termed ice-ball formation. Wang et al. (2) reported that cavitation was the most common postablation finding and approximately 17% of the ablated lesions disappeared at 12 months. The remaining lesions without local recurrence might decrease in size or maintain a stable size. Positron emission tomography-CT might also have a promising role in the estimation of the therapeutic effects, in the differentiation of tumor necrosis and viable tumor proportion (4).
Yamauchi et al. (7) reported that the overall survival rates after cryoablation for inoperable lung cancer were 95% at 1 year, 88% at 2 years, and 88% at 3 years. The disease-free survival percentages were 91, 78, and 67% at 1, 2, and 3 years, respectively. According to a recent report, the efficacy of lung tumor cryoablation is comparable with RFA and sublobar resection (3).
There are several reports of cryoablation as an alternative treatment option for lung lesions in Korea. Lee et al. (8) reported a case of successful cryoablation performed for treatment of recurrent lung cancer. The patient was not a candidate for surgery because he had already undergone right upper lobe resection and left upper lobe resection due to bilateral lung cancers. Treated lesion showed cavitary necrosis as in our case and maintained stable for 2 years during follow up. However, not all reports showed promising success rates. Park et al. (9) reported the result of cryoablations performed for 14 lung malignancies, with complete ablation achieved in only 4 cases (35.7%). The authors mentioned that the results of percutaneous cryotherapy were not as satisfactory as expected, but still, cryotherapy could be performed safely in inoperable patients with lung malignancy and it might lengthen their survival period. Recently, Kim et al. (10) reported that cryoablation could be used as a new treatment method for ground-glass nodules.
We report a case of complete necrosis of recurrent lung cancer after resection and radiotherapy. Percutaneous ablation is a minimally invasive therapy and it is generally safe and well tolerated, with low rates of morbidity and very low procedure-related mortality. The advantages of cryoablation over radiofrequency ablation include the following: it can achieve larger tumor ablation volumes, allows for the use of multiple applicators, provides a highly visible ablation zone, and causes less procedural pain due to the analgesic effect of freezing. Compared with heat-based thermal ablation therapies, cryoablation preserves the collagenous tissue and cellular architecture, which makes it a safer option near vasculature or bronchi. A growing body of literature describes the successful use of cryoablation in the treatment of malignancies in the lung, and application of percutaneous cryoablation may represent a new promising effective treatment option for lung tumors in patients who are not candidates of surgical resection.
Figures and Tables
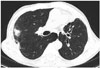 | Fig. 1Chest CT with lung window reveals a 1.5 cm sized spiculated nodule with an air bronchogram in the right upper lobe. The nodule was proved to be metastatic adenocarcinoma by percutaneous needle biopsy.CT = computed tomography
|
References
1. Ahmed M, Brace CL, Lee FT Jr, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology. 2011; 258:351–369.
2. Wang H, Littrup PJ, Duan Y, Zhang Y, Feng H, Nie Z. Thoracic masses treated with percutaneous cryotherapy: initial experience with more than 200 procedures. Radiology. 2005; 235:289–298.
3. Zemlyak A, Moore WH, Bilfinger TV. Comparison of survival after sublobar resections and ablative therapies for stage I non-small cell lung cancer. J Am Coll Surg. 2010; 211:68–72.
4. Zhang X, Tian J, Zhao L, Wu B, Kacher DS, Ma X, et al. CT-guided conformal cryoablation for peripheral NSCLC: initial experience. Eur J Radiol. 2012; 81:3354–3362.
5. Pusceddu C, Sotgia B, Fele RM, Melis L. CT-guided thin needles percutaneous cryoablation (PCA) in patients with primary and secondary lung tumors: a preliminary experience. Eur J Radiol. 2013; 82:e246–e253.
6. Inoue M, Nakatsuka S, Yashiro H, Ito N, Izumi Y, Yamauchi Y, et al. Percutaneous cryoablation of lung tumors: feasibility and safety. J Vasc Interv Radiol. 2012; 23:295–302. quiz 305.
7. Yamauchi Y, Izumi Y, Hashimoto K, Yashiro H, Inoue M, Nakatsuka S, et al. Percutaneous cryoablation for the treatment of medically inoperable stage I non-small cell lung cancer. PLoS One. 2012; 7:e33223.
8. Lee SH, Kim KT, Chung JH, Jo SB, Youn HS, Son HS. Percutaneous cryoablation of lung cancer in high risk patients. Korean J Thorac Cardiovasc Surg. 2006; 39:953–956.
9. Park EH, Jin GY, Han YM, Lee YC, Kwon KS. Percutaneous cryotherapy for inoperable lung malignancy. J Korean Soc Radiol. 2012; 66:427–435.
10. Kim KY, Jin GY, Han YM, Lee YC, Jung MJ. Cryoablation of a small pulmonary nodule with pure ground-glass opacity: a case report. Korean J Radiol. 2015; 16:657–661.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download