Abstract
Purpose
To evaluate the incidence, severity, and risk factors of adverse drug reactions (ADR) to intravenous administration of nonionic iodinated contrast media in computed tomography (CT), and to determine the recurrence rate after premedication in patients with a previous history of ADR.
Materials and Methods
We prospectively recorded all ADR to intravenous CT contrast media in 32313 consecutive outpatients (54572 cases) who underwent contrast enhanced CT examinations. Clinical report forms and electronic medical records were reviewed to search for the incidence of ADR, treatment, and clinical outcome of patients. The risk factors of ADR to CT contrast media (age, sex, history of previous ADR, season) were evaluated using statistical analysis.
Results
Of the 54572 cases, a total of 191 (0.35%) had adverse reactions. Of the 191 cases, 157 (82%) were categorized as mild reactions, 29 (15%) were moderate, and 5 (3%) were severe. A total of 165 (86.4%) cases had acute adverse reactions (which occurred within 1 hour after administration), while 26 (13.6%) had delayed adverse reactions (occurred 1 hour after the administration). The rate of ADR was significantly higher in females [relative risk (RR) = 2.05, 95% confidence interval (CI) 1.53–2.75], patients under the age of 60 years (RR = 1.45, 95% CI 1.07–1.98), patients with a history of previous ADR (RR = 6.51, 95% CI 3.13–13.57), and in the spring season (RR = 1.44, 95% CI 1.07–1.95). The recurrence rate after premedication in patients with previous ADR to CT contrast media was 3.2% (8/247). No deaths occurred that were attributed to the contrast media.
Figures and Tables
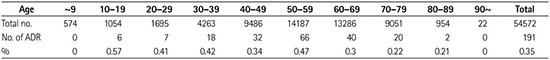
Fig. 1
Comparison of adverse drug reactions to CT contrast media by age. Bars represent mean values and error lines represent standard errors (Appendix 1).

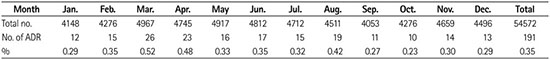
Fig. 2
Comparison of adverse drug reactions to CT contrast media by month of the year. Bars represent mean values and error lines represent standard errors (Appendix 2).

Table 1
Patients' Demographics (n = 54572)

Table 2
Type, Severity and Symptoms of ADR to CT Contrast Media

Table 3
Risks of ADR to CT Contrast Media

Table 4
Treatment of ADR to CT Contrast Media

Acknowledgments
This Work (RPP-2015-022) was supported by the fund of Research Promotion Program, Gyeongsang National University, 2015.
References
1. Namasivayam S, Kalra MK, Torres WE, Small WC. Adverse reactions to intravenous iodinated contrast media: a primer for radiologists. Emerg Radiol. 2006; 12:210–215.
2. Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology. 1990; 175:621–662.
3. Thomsen HS, Bush WH Jr. Adverse effects of contrast media: incidence, prevention and management. Drug Saf. 1998; 19:313–324.
4. ACR Committee on Drugs and ContrastMedia. ACR manual on contrast media version 10.1. Patient Selection And Preparation Strategies. 2015. 9–15. Accessed Dec 10, 2015. Available at: http://www.acr.org/~/media/37D84428BF1D4E1B9A3A2918DA9E27A3.pdf.
5. ACR Committee on Drugs and ContrastMedia. ACR manual on contrast media version 10.1. Table 3. Categories of reactions. 2015. 103. Accessed Dec 10, 2015. Available at: http://www.acr.org/~/media/37D84428BF1D4E1B9A3A2918DA9E27A3.pdf.
6. Christiansen C. X-ray contrast media--an overview. Toxicology. 2005; 209:185–187.
7. Wang CL, Cohan RH, Ellis JH, Caoili EM, Wang G, Francis IR. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am J Roentgenol. 2008; 191:409–415.
8. Li X, Chen J, Zhang L, Liu H, Wang S, Chen X, et al. Clinical observation of the adverse drug reactions caused by nonionic iodinated contrast media: results from 109,255 cases who underwent enhanced CT examination in Chongqing, China. Br J Radiol. 2015; 88:20140491.
9. Ho J, Kingston RJ, Young N, Katelaris CH, Sindhusake D. Immediate hypersensitivity reactions to IV non-ionic iodinated contrast in computed tomography. Asia Pac Allergy. 2012; 2:242–247.
10. Mortelé KJ, Oliva MR, Ondategui S, Ros PR, Silverman SG. Universal use of nonionic iodinated contrast medium for CT: evaluation of safety in a large urban teaching hospital. AJR Am J Roentgenol. 2005; 184:31–34.
11. Thomsen HS, Morcos SK. ESUR. ESUR guidelines on contrast media. Abdom Imaging. 2006; 31:131–140.
12. Kopp AF, Mortele KJ, Cho YD, Palkowitsch P, Bettmann MA, Claussen CD. Prevalence of acute reactions to iopromide: postmarketing surveillance study of 74,717 patients. Acta Radiol. 2008; 49:902–911.
13. Davenport MS, Cohan RH, Caoili EM, Ellis JH. Repeat contrast medium reactions in premedicated patients: frequency and severity. Radiology. 2009; 253:372–379.
14. Kim SH, Lee SH, Lee SM, Kang HR, Park HW, Kim SS, et al. Outcomes of premedication for non-ionic radio-contrast media hypersensitivity reactions in Korea. Eur J Radiol. 2011; 80:363–367.
15. Bellin MF, Stacul F, Webb JA, Thomsen HS, Morcos SK, Almén T, et al. Late adverse reactions to intravascular iodine based contrast media: an update. Eur Radiol. 2011; 21:2305–2310.
16. Almén T. The etiology of contrast medium reactions. Invest Radiol. 1994; 29:Suppl 1. S37–S45.
17. Prelog M. Aging of the immune system: a risk factor for autoimmunity? Autoimmun Rev. 2006; 5:136–139.
18. Narita S, Goldblum RM, Watson CS, Brooks EG, Estes DM, Curran EM, et al. Environmental estrogens induce mast cell degranulation and enhance IgE-mediated release of allergic mediators. Environ Health Perspect. 2007; 115:48–52.
19. Mikkonen R, Vehmas T, Granlund H, Kivisaari L. Seasonal variation in the occurrence of late adverse skin reactions to iodine-based contrast media. Acta Radiol. 2000; 41:390–393.
20. Palmiere C, Reggiani Bonetti L. Risk factors in fatal cases of anaphylaxis due to contrast media: a forensic evaluation. Int Arch Allergy Immunol. 2014; 164:280–288.
21. Nagamoto M, Gomi T, Terada H, Terada S, Kohda E. Evaluation of the acute adverse reaction of contrast medium with high and moderate iodine concentration in patients undergoing computed tomography. Radiat Med. 2006; 24:669–674.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download