INTRODUCTION
Adenomyoepithelioma (AME) is an uncommon disease entity representing biphasic proliferation of ductal epithelial and myoepithelial cells. AME is generally considered as benign. Malignant AMEs are rarely reported. There are various imaging features of AME. In this case report, the mass showed complex solid and cystic appearance, suggesting suspicious abnormality. To the best of our knowledge, there are only a few English written literatures about hemorrhagic AME.
CASE REPORT
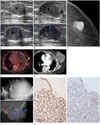
An 85-year-old woman was referred to our hospital due to multiple lung nodules confirmed as adenocarcinoma on percutaneous needle biopsy. On chest computed tomography (CT) scan, a 1.4 cm sized mass in the left breast was incidentally detected. She had no palpable mass in the breast. Ultrasonography (US) revealed a 1.4 cm sized, ovoid shaped, circumscribed, and isoechoic mass with internal cystic portion having increased vascularity in the left breast 3 o'clock. The mass was presumed as Breast Imaging Reporting and Data System (BI-RADS) category 4B (Fig. 1A). US-guided core needle biopsy was performed and the diagnosis was confirmed as papilloma.
On follow-up chest CT for lung cancer, the breast mass was increased in size. One year later, US demonstrated a 2.5 cm sized complex solid and cystic mass with increased vascularity in the left breast (Fig. 1B). Mammography revealed a 2.5 cm sized, oval shaped hyper-dense mass with partially indistinct margin in the mid-outer aspect of the left breast (Fig. 1C). Positron emission tomography (PET)/CT scan showed hypermetabolic uptake in the left breast mass. Pleural nodules and effusion due to non-small cell lung cancer were also seen (Fig. 1D). The maximum standardized uptake value (SUVmax) of the breast mass was 9.8. US-guided biopsy was performed once again and it was pathologically confirmed as intraductal papilloma at that time.
Although the breast mass had grown during the follow-up, the patient was reluctant to undergo resection due to old age. Chest CT taken three years after the second biopsy showed a 9 cm sized lobulated mass in the left breast. The mass had irregular and nodular septa with enhancement accompanied by adjacent skin thickening (Fig. 1E).
The mass recently caused bleeding. Physical examination revealed a 13 cm sized round shaped firm and non-tender mass in the left breast. No axillary lymphadenopathy was detected. US confirmed a 13 cm sized, irregular shaped, circumscribed, complex solid and cystic mass in the left breast (Fig. 1F). Most of the mass was non-solid component with internal echogenic floating material. The mass had hyperechoic solid mural nodules and septa. These mural nodules showed increased vascularity on color Doppler exam. This lesion was reassessed as BI-RADS category 4B in spite of its increase in size. The radiologist considered the mass has increased in diameter slowly for several years without evidence of metastatic lymphadenopathy despite of its huge size The change in size was mainly due to increased amount of non-solid component, suggestive of hemorrhage or necrosis. Core needle biopsy was performed the third time and the pathologic diagnosis of intraductal papilloma was confirmed again.
After that, mastectomy was performed due to profuse bloody nipple discharge. On immunohistochemical staining, tumor cells were positive for cytokeratin 19 and p63, supporting the diagnosis of AME (Fig. 1G, H).
DISCUSSION
AME is considered as an unusual breast tumor representing biphasic proliferation of ductal epithelial and myoepithelial cells. AME usually occurs in elderly women. It very rarely occurs in men (1). Most AMEs are benign. Malignant AMEs are rarely reported. Malignant change can occur in the myoepithelial or epithelial or both components. Malignant change is more common in the epithelial component (2). Both benign and malignant lesions might have local invasion, recurrence, and metastasis.
Mammographic findings of AME are nonspecific in previous literatures. AME tumors have been reported as focal asymmetry or oval or irregular isodense mass with circumscribed or indistinct or microlobulated margins (345). A few cases of AME are accompanied by calcification (356). Architectural distortion is uncommon. It is associated with malignant AME (6).
On US, hypoechoic solid masses with irregular shape, microlobulated margin, non-parallel orientation, and increased peripheral vascularity are common findings. These features occur in more than half of the cases reported (47). AME may have complex echogenicity or angular margin with some ductal ectasia and posterior acoustic enhancement (67).
Several reports on magnetic resonance imaging of benign AME have described oval smooth mass with persistent pattern on kinetic curve, round shape mass with spiculated border and plateau pattern on kinetic curve, or irregular spiculated mass with heterogeneous enhancement and washout pattern on kinetic curve (36). In recent literatures, three cases of benign AME show low to iso signal intensity on T1-weighted image, slightly high signal intensity on T2-weighted image, and homogeneous enhancement with plateau or persistent pattern on kinetic curve (5). A case of malignant AME is reported to have washout pattern on kinetic curve and relatively low apparent diffusion coefficient (ADC) value (5).
Two cases of adenomyoepithelial tumors with PET/CT scan have been reported. One case showed no fluorodeoxyglucose (FDG) uptake while the other showed FDG uptake with a SUV-max of 4.9 (6). In the present case, the patient showed relatively high FDG uptake with a SUVmax of 9.8.
Although breast AME with cystic change is rare, it has been reported (68). Although there are a few reports about predominantly cystic or intracystic AME, none of them have reported hemorrhagic component as shown in the current case.
Differential diagnosis of AME includes invasive ductal cancer with necrosis or hemorrhage, malignant phyllodes tumor, and intraductal or intracystic papilloma.
The diagnosis of AME is very challenging due to its rarity and nonspecific clinical and radiologic features. The mass may be detected as a palpable mass or as an incidental finding. Rapid enlargement is suggestive of malignancy (3).
In the present case, previous biopsy results were papilloma (three times). The papillary lesions appeared well circumscribed with round or oval shape mass on mammography, typically in the retroareolar regions. Sonographic appearances can be divided into two broad categories (intraductal mass, complex cystic, and solid mass). Posterior acoustic enhancement is commonly seen in both solid and cystic components. On color Doppler exam, highly vascular pedicle can be detected. These imaging features may overlap with those of AME (9).
Regarding cytology findings, AME may also be confused with other neoplasms due to its various hypercellular features. Most AMEs show papillary structure considered as a variant of intraductal papilloma (8). AME consists of cuboidal to columnar epithelial-lined tubules surrounded by a layer of myoepithelial cells with prominent clear cytoplasm. Papillomas are characterized by an arborescent proliferation of epithelial and myoepithelial cells with fibrovascular cores protruding into ductal lumens. The differentiation of papilloma with prominent myoepithelial cells from AME is dependent on the architecture, pattern, and degree of myoepithelial proliferation. A truly benign-appearing, biphasic proliferation, more solids than ordinary papilloma with a distinct proliferating component of myoepithelial cells should be checked as possible AME. Immunohistochemical expression of myoepithelial markers such as calponin, p63, and smooth muscle actin can be helpful for a diagnosis of AME (10). In the present case, core needle biopsy was done for the solid component three times. However, those samples may not represent the mass. Even if the core needle biopsy is performed, it can still be difficult to diagnose AME and predict the malignant potential.
Treatment of benign AME is wide local excision to prevent local recurrence. Adjuvant chemotherapy may be administered if there is malignant potential. Long-term follow-up is recommended.
In conclusion, the imaging features of AMEs of the breast are nonspecific. They overlap with suspicious features. Although rare AMEs should be considered as a differential diagnosis of complex solid and cystic breast mass. Concordance between imaging and pathologic findings must be well established with core needle biopsy. The radiologist should also be aware of the potential of local recurrence of AME.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download