Abstract
Purpose
The aim of this study was to evaluate added value and diagnostic threshold value of thyroglobulin measurement in the fine-needle aspiration washout for detecting cervical lymph node metastasis from papillary thyroid cancer on pre and postoperative patients.
Materials and Methods
Total 219 cervical lymph nodes from 180 patients with papillary thyroid cancer were evaluated for fine needle aspiration cytology and thyroglobulin in fine needle aspiration (FNA-Tg), using immunometric chemiluminescent assay. Eighty-six patients were preoperative and remaining 94 patients were on follow up after total thyroidectomy. Final diagnoses were made on pathology of dissected lymph nodes or follow-up examination for at least 12 months.
Results
One hundred and twelve metastatic lymph nodes were finally confirmed in 94 patients out of total 180 patients. Sensitivity of FNA-Tg was 99.1, 98.21, 97.20%, respectively with threshold level at 1, 10, serum Tgng/mL, which were higher sensitivity of fine needle aspiration. Combined FNA and FNA-Tg with threshold at 1, 10, 100 ng/mL raised sensitivity and specificity to 100%, respectively. All 6 lymph nodes that were false negative on FNA were correctly diagnosed as metastasis on FNA-Tg with threshold of 1, 10, 100, and serum thyroglobulin. FNA-Tg with threshold level at 100 ng/mL combined FNA showed highest sensitivity (100%) and specificity (97.56%) on preoperative patient groups among the 1, 10, 100, serum Tg threshold value. But, FNA only showed adequately high sensitivity (100%) and specificity (96.96%) on postoperative patient groups. Using receiver operating characteristic curve (ROC) curve analysis, cut off value was 57.69 in total patient, 78.66 in preoperative patient, and 32.81 in postoperative patient.
Conclusion
FNA-Tg combined with FNA showed excellent sensitivity and specificity. FNA-Tg showed very high sensitivity and specificity at threshold level 78.66 ng/mL in preoperative patients, but FNA-Tg had less benefit on the postoperative patient group, having high sensitivity and specificity with FNA alone.
Figures and Tables
Fig. 1
Metastatic cystic cervical lymph node. Preoperative ultrasound in a 38-year-old man shows a 1.3 cm sized solid and calcified nodule in right thyroid and 3.3 cm cystic lymph node (caliber) at the level IV of the left neck. Ultrasound guided fine needle aspiration cytology reveals atypical cells; but the value of thyroglobulin in the fine needle aspiration washout is 132 ng/mL. The thyroid nodule is confirmed as papillary thyroid cancer and lymph node is confirmed pathologically as metastatic lymph node on thyroidectomy and lymph node dissection.
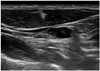
Fig. 2
Metastatic microcalcified cervical lymph node. Preoperative ultrasound in a 24-year-old woman shows a 2 cm sized microcalcified nodule in right thyroid and 0.7 cm microcalcified lymph node (caliber) at the level 3 of the right neck. Ultrasound guided fine needle aspiration cytology reveals inadequate specimen; but the value of thyroglobulin in the fine needle aspiration washout is 203 ng/mL. The thyroid nodule is confirmed as papillary thyroid cancer and lymphnode is confirmed pathologically as metastatic lymph node at thyroidectomy and lymph node dissection.
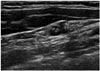
Fig. 3
The values of FNA-Tg wash-out of metastatic lymph nodes (group1) and non-metastatic lymph nodes (group 2).
FNA-Tg = thyroglobulin in fine needle aspiration

Fig. 4
The values of FNA-Tg wash-out/serum thyroglobulin in metastatic lymph nodes and benign lymph nodes.
FNA-Tg = thyroglobulin in fine needle aspiration

Fig. 5
The value of FNA-Tg wash-out of metastatic lymph nodes in preoperative metastatic lymph nodes and postoperative metastatic lymph nodes.
FNA-Tg = thyroglobulin in fine needle aspiration

Fig. 6
The value of serum thyroglobulin (Tg) in preoperative metastatic lymph nodes and postoperative metastatic lymph nodes.

Fig. 7
Receiver operator characteristics for FNA-Tg in this study.
A. Receiver operator characteristics for FNA-Tg in the total patient cohort of this study. The area under the curve is 0.988.
B. Receiver operator characteristics for FNA-Tg in this study in preoperative patients. The area under the curve is 0.988.
C. Receiver operator characteristics for FNA-Tg in this study in post-operative patients. The area under the curve is 0.988.
FNA-Tg = thyroglobulin in fine needle aspiration

Table 1
Sensitivity and Specificity of FNAC Versus FNA-Tg with Different Threshold

Table 2
Sensitivity and Specificity of FNA Versus Combined FNAC and FNA-Tg with Different Threshold

Table 3
Sensitivity and Specificity of FNA Versus FNA-Tg with Different Threshold on Pre-Operative and Post-Operative Groups

Table 4
Sensitivity and Specificity of FNA Versus Combined FNAC and FNA-Tg with Different Threshold on Pre-Operative and Post-Operative Groups

References
1. Cunha N, Rodrigues F, Curado F, Ilh?u O, Cruz C, Naidenov P, et al. Thyroglobulin detection in fine-needle aspirates of cervical lymph nodes: a technique for the diagnosis of metastatic differentiated thyroid cancer. Eur J Endocrinol. 2007; 157:101–107.
2. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26:1–133.
3. Shaha AR, Shah JP, Loree TR. Patterns of nodal and distant metastasis based on histologic varieties in differentiated carcinoma of the thyroid. Am J Surg. 1996; 172:692–694.
4. DeGroot LJ. Long-term impact of initial and surgical therapy on papillary and follicular thyroid cancer. Am J Med. 1994; 97:499–500.
5. McConahey WM, Hay ID, Woolner LB, van Heerden JA, Taylor WF. Papillary thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc. 1986; 61:978–996.
6. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006; 16:109–142.
7. Takashima S, Sone S, Nomura N, Tomiyama N, Kobayashi T, Nakamura H. Nonpalpable lymph nodes of the neck: assessment with US and US-guided fine-needle aspiration biopsy. J Clin Ultrasound. 1997; 25:283–292.
8. Frasoldati A, Valcavi R. Challenges in neck ultrasonography: lymphadenopathy and parathyroid glands. Endocr Pract. 2004; 10:261–268.
9. Frasoldati A, Toschi E, Zini M, Flora M, Caroggio A, Dotti C, et al. Role of thyroglobulin measurement in fine-needle aspiration biopsies of cervical lymph nodes in patients with differentiated thyroid cancer. Thyroid. 1999; 9:105–111.
10. Kim E, Park JS, Son KR, Kim JH, Jeon SJ, Na DG. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid. 2008; 18:411–418.
11. Snozek CL, Chambers EP, Reading CC, Sebo TJ, Sistrunk JW, Singh RJ, et al. Serum thyroglobulin, high-resolution ultrasound, and lymph node thyroglobulin in diagnosis of differentiated thyroid carcinoma nodal metastases. J Clin Endocrinol Metab. 2007; 92:4278–4281.
12. Cunha N, Rodrigues F, Curado F, Ilhéu O, Cruz C, Naidenov P, et al. Thyroglobulin detection in fine-needle aspirates of cervical lymph nodes: a technique for the diagnosis of metastatic differentiated thyroid cancer. Eur J Endocrinol. 2007; 157:101–107.
13. Mikosiński S, Pomorski L, Oszukowska L, Makarewicz J, Adamczewski Z, Sporny S, et al. The diagnostic value of thyroglobulin concentration in fine-needle aspiration of the cervical lymph nodes in patients with differentiated thyroid cancer. Endokrynol Pol. 2006; 57:392–395.
14. Boi F, Baghino G, Atzeni F, Lai ML, Faa G, Mariotti S. The diagnostic value for differentiated thyroid carcinoma metastases of thyroglobulin (Tg) measurement in washout fluid from fine-needle aspiration biopsy of neck lymph nodes is maintained in the presence of circulating anti-Tg antibodies. J Clin Endocrinol Metab. 2006; 91:1364–1369.
15. Cignarelli M, Ambrosi A, Marino A, Lamacchia O, Campo M, Picca G, et al. Diagnostic utility of thyroglobulin detection in fine-needle aspiration of cervical cystic metastatic lymph nodes from papillary thyroid cancer with negative cytology. Thyroid. 2003; 13:1163–1167.
16. Pacini F, Fugazzola L, Lippi F, Ceccarelli C, Centoni R, Miccoli P, et al. Detection of thyroglobulin in fine needle aspirates of nonthyroidal neck masses: a clue to the diagnosis of metastatic differentiated thyroid cancer. J Clin Endocrinol Metab. 1992; 74:1401–1404.
17. Uruno T, Miyauchi A, Shimizu K, Tomoda C, Takamura Y, Ito Y, et al. Usefulness of thyroglobulin measurement in fine-needle aspiration biopsy specimens for diagnosing cervical lymph node metastasis in patients with papillary thyroid cancer. World J Surg. 2005; 29:483–485.
18. Miseikyte-Kaubriene E, Trakymas M, Ulys A. Cystic lymph node metastasis in papillary thyroid carcinoma. Medicina (Kaunas). 2008; 44:455–459.
19. Jeon SJ, Kim E, Park JS, Son KR, Baek JH, Kim YS, et al. Diagnostic benefit of thyroglobulin measurement in fine-needle aspiration for diagnosing metastatic cervical lymph nodes from papillary thyroid cancer: correlations with US features. Korean J Radiol. 2009; 10:106–111.
20. Grebe SK, Hay ID. Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am. 1996; 5:43–63.
21. Baskin HJ. Detection of recurrent papillary thyroid carcinoma by thyroglobulin assessment in the needle washout after fine-needle aspiration of suspicious lymph nodes. Thyroid. 2004; 14:959–963.
22. Torréns JI, Burch HB. Serum thyroglobulin measurement. Utility in clinical practice. Endocrinol Metab Clin North Am. 2001; 30:429–467.
23. Ustün M, Risberg B, Davidson B, Berner A. Cystic change in metastatic lymph nodes: a common diagnostic pitfall in fine-needle aspiration cytology. Diagn Cytopathol. 2002; 27:387–392.
24. Tseng FY, Hsiao YL, Chang TC. Cytologic features of metastatic papillary thyroid carcinoma in cervical lymph nodes. Acta Cytol. 2002; 46:1043–1048.
25. Ahuja S, Ernst H, Lenz K. Papillary thyroid carcinoma: occurrence and types of lymph node metastases. J Endocrinol Invest. 1991; 14:543–549.
26. Monchik JM, De Petris G, De Crea C. Occult papillary carcinoma of the thyroid presenting as a cervical cyst. Surgery. 2001; 129:429–432.
27. Verge J, Guixá J, Alejo M, Basas C, Quer X, De Castro J, et al. Cervical cystic lymph node metastasis as first manifestation of occult papillary thyroid carcinoma: report of seven cases. Head Neck. 1999; 21:370–374.
28. Matsuda M, Nagumo S, Koyama H, Wada A. Occult thyroid cancer discovered by fine-needle aspiration cytology of cervical lymph node: a report of three cases. Diagn Cytopathol. 1991; 7:299–303.
29. Levy I, Barki Y, Tovi F. Cystic metastases of the neck from occult thyroid adenocarcinoma. Am J Surg. 1992; 163:298–300.
30. Hwang CF, Wu CM, Su CY, Cheng L. A long-standing cystic lymph node metastasis from occult thyroid carcinoma--report of a case. J Laryngol Otol. 1992; 106:932–934.
31. Kim MJ, Kim EK, Kim BM, Kwak JY, Lee EJ, Park CS, et al. Thyroglobulin measurement in fine-needle aspirate washouts: the criteria for neck node dissection for patients with thyroid cancer. Clin Endocrinol (Oxf). 2009; 70:145–151.
32. Gholamrezanezhad A, Saghari M, Mirpour S, Beiki D, Tarbiat A, Javan S, et al. [On-levothyroxine measurement of thyroglobulin is not a reliable test for the follow-up of patients at high risk for remnant/recurrent differentiated thyroid carcinoma]. Endokrynol Pol. 2007; 58:100–104.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download