Abstract
Undifferentiated pleomorphic sarcoma, known as malignant fibrous histiocytoma, arising from the wall of the aorta is an extremely rare tumor. We report computed tomography and magnetic resonance imaging findings of a 67-year-old male patient with histopathologically proven undifferentiated pleomorphic sarcoma arising from the wall of the descending thoracic aorta. To the best of our knowledge, this is the first report of such a case in Korea. Knowledge of imaging findings of undifferentiated pleomorphic sarcoma will be helpful for accurate diagnosis and appropriate management.
Primary sarcomas of the great vessels are extremely uncommon tumors, with aortic sarcoma being the rarest type. They tend to occur in the thoracic aorta, followed by the abdominal aorta and the thoracoabdominal aorta. They have a propensity for metastases and they cause acute arterial embolism (1).
Undifferentiated pleomorphic sarcoma, also called malignant fibrous histiocytoma, has been defined as a group of pleomorphic, high-grade sarcomas that fail to show any line of differentiation using currently available ancillary techniques (2). To the best of our knowledge, no fewer than 24 cases of undifferentiated pleomorphic sarcoma originating from the aorta have been reported in the worldwide literature, but imaging findings have been rarely reported (34567).
Herein, we describe the case of a 67-year-old male who had a primary undifferentiated pleomorphic sarcoma of the descending thoracic aorta with an emphasis on computed tomography (CT) and magnetic resonance imaging (MRI) findings.
A 67-year-old Russian male presented with a 5-month history of epigastric and back pain. The patient had a history of diabetes mellitus and hypertension. Laboratory studies revealed abnormal liver function test (gamma-glutamyl transpeptidase: 143 IU/L, alkaline phosphatase: 448 IU/L) and increased C-reactive protein (1.98 mg/dL). Electrocardiogram showed normal sinus rhythm. Contrast enhanced abdomen and chest CT (64-slice multidetector CT, GE Healthcare, LightSpeed VCT, Milwaukee, WI, USA) were performed. Abdomen CT showed no significant findings, and contrast-enhanced CT of the patient's chest showed a heterogeneously enhancing lobulated mass encasing the right aspect of the descending thoracic aorta, extending from the T6 to T8 level, and measuring 1.8 × 3.5 × 3.2 cm in size (Fig. 1A, B). The mass occupied 20% of the aortic lumen, and the intimal layer of the aorta was intact. Thoracic MRI on a 1.5 T system (Intera, Philips Healthcare, Best, the Netherlands) was subsequently performed. The mass showed iso-signal intensity on the T1-weighted image and strong peripheral enhancement with irregular heterogeneously enhancing foci within the lesion after contrast enhancement (Fig. 1C, D). Fat plane between the mass and adjacent lung was preserved, and there was no invasion into the adjacent vertebral bodies or ribs. On the T2-weighted image, the mass showed heterogeneously high signal intensity (Fig. 1E). Based on the constellation of the described imaging appearance, the mass was considered to be a sarcoma arising from the posterior mediastinum.
At thoracotomy, a 2 × 4 cm sized mass invading the descending thoracic aorta was noted (Fig. 1F), and excision of the mass and replacement of the thoracic aorta using artificial vascular graft were performed. Histopathological evaluation revealed that the mass directly involved the adventitia of the aorta and periaortic soft tissue with hemorrhage and necrosis. Hematoxylin and eosin (× 600) (Fig. 1G) stain demonstrated fascicles of spindle cells with marked pleomorphism, prominent nucleoli, and high mitotic rate. Immunohistochemical stains showed immunonegativity for CD34 and S100, but immunopositivity for smooth muscle actin.
One week later, positron emission tomography-computed tomography (PET-CT) revealed hypermetabolic paraaortic lymph node [standardized uptake value (SUV) = 3.4], hypermetabolic nodular lesion in the distal thoracic paraaortic area (SUV = 3.8), and mildly hypermetabolic bilateral hilar lymph nodes (SUV = 3.1–3.6). One week later, excision of the paraaortic lymph node and adventitial tissue via exploratory thoracotomy was performed. Histopathological evaluation revealed reactive lymph node hyperplasia and acute and chronic inflammation without definite malignant cells.
The overall findings were consistent with a primary undifferentiated pleomorphic sarcoma of the descending thoracic aorta. The patient was subsequently referred to another Russia-based hospital for postoperative treatment.
Undifferentiated pleomorphic sarcoma occurring in the aorta is known to be a rare entity. Among the 24 cases of undifferentiated pleomorphic sarcoma occurring in the aorta in the English literature, this tumor was more common in males (male:female ratio, 13:11) and mean age of the patients was 61.6 years. Undifferentiated pleomorphic sarcoma of the aorta occurs most commonly in the descending thoracic aorta (62.5%), followed by the abdominal aorta (33.3%). The common clinical presentation is lower extremity pain related to an embolic event, abdominal pain, and back pain. Metastasis occurs most frequently in the lung, bone and adrenal gland. The interest in this case is derived from the typical clinical presentation and the location of undifferentiated pleomorphic sarcoma in the aorta (34567).
No specific imaging findings are known to differentiate the various subtypes of sarcomas, and some radiologic findings of undifferentiated pleomorphic sarcoma occurring in the aorta have been reported. CT demonstrated a large, infiltrating, minimally enhancing soft-tissue mass in the thoracic aorta showing rapid growth (34). Tejada et al. (5) reported that myxoid malignant fibrous histiocytoma appeared as a lobulated luminal exophytic mass in the distal thoracic aorta without extra-aortic extension. Higgins et al. (6) reported a high signal intensity mass in the lower thoracic aorta with an intraluminal filling defect and possible extension through the aortic wall, on the T2 weighted image. There is no report on PET-CT findings of undifferentiated pleomorphic sarcoma occurring in the aorta, and Kim and Kim (8) reported a case of a huge perihepatic undifferentiated pleomorphic sarcoma with intense hypermetabolic activity in the peripheral solid portion based on PET-CT. Similarly, in our case, the lesion showed heterogeneous enhancement on CT and strong peripheral enhancement with irregular heterogeneous foci within the lesion on MRI. Based on the constellation of these imaging features, the mass was considered as a sarcoma.
Differentiation between undifferentiated pleomorphic sarcoma and atherosclerotic disease such as ulcer like projection with aneurysmal formation can be difficult on CT. Irregularity of the aortic lumen and the circumferential extent of the tumor may be characteristic CT findings of a mural based aortic tumor (47). In the present case, the lesion showed eccentric aortic wall thickening and the intima was preserved with smooth margin on CT, which was initially mistaken for an intramural hematoma from an aortic aneurysm. But the enhancement pattern of the mass on CT and MRI was helpful for differential diagnosis. Also, we believe that the findings of PET-CT might be helpful for diagnosis of sarcoma because of the hypermetabolic activity of the lesion.
The choice of treatment for undifferentiated pleomorphic sarcoma is local surgical resection (3567). The small number of cases assessed so far does not allow ultimate evaluation of the role of adjuvant chemotherapy and radiotherapy (4).
In summary, we report an unusual case of undifferentiated pleomorphic sarcoma of the descending thoracic aorta presenting with epigastric and back pain. When confronted with an heterogeneously enhancing lesion of the thoracic aorta that shows iso-signal intensity on the T1-weighted image with strong peripheral enhancement with irregular heterogeneously enhancing foci within the lesion and high signal intensity on the T2-weighted image, undifferentiated pleomorphic sarcoma should be included in the differential diagnosis.
Figures and Tables
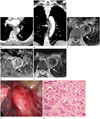 | Fig. 1A 67-year-old male with undifferentiated pleomorphic sarcoma of the descending thoracic aorta.Axial contrast-enhanced computed tomography image (A) shows a heterogeneously enhancing lobulated mass-like lesion encasing the descending thoracic aorta and measuring 1.8 × 3.5 × 3.2 cm in size (white arrow). Coronal (B) reformatted image obtained in the venous phase (arrow) shows a lesion extending from the T6 to T8 level (white arrow). (C) The mass shows intermediate signal intensity on the T1 weighted image (white arrow) (D), strong peripheral enhancement with irregular heterogeneously enhancing foci within the lesion (white arrow), and heterogeneous high signal intensity on the T2 weighted image (white arrow) (E).
F. At thoracotomy, an approximately 2 × 4 cm sized mass invading the thoracic aorta (white arrow) is noted.
G. Hematoxylin and eosin (× 600) stain demonstrates fascicles of spindle cells with marked pleomorphism, prominent nucleoli, and high mitotic rate.
|
References
1. Rusthoven CG, Liu AK, Bui MM, Schefter TE, Elias AD, Lu X, et al. Sarcomas of the aorta: a systematic review and pooled analysis of published reports. Ann Vasc Surg. 2014; 28:515–525.
2. Goldblum JR. An approach to pleomorphic sarcomas: can we subclassify, and does it matter? Mod Pathol. 2014; 27:Suppl 1. S39–S46.
3. Tatsu K, Kamata S, Kasahara K. Rapidly progressive descending aortic pseudoaneurysm resulting from primary malignant fibrous histiocytoma. Gen Thorac Cardiovasc Surg. 2012; 60:498–500.
4. Utsunomiya D, Ikeda O, Ideta I, Hirayama T, Yamashita Y, Kamio T. Malignant fibrous histiocytoma arising from the aortic wall mimicking a pseudoaneurysm with ulceration. Circ J. 2007; 71:1659–1661.
5. Tejada E, Becker GJ, Waller BF. Malignant myxoid emboli as the presenting feature of primary sarcoma of the aorta (myxoid malignant fibrous histiocytoma): a case report and review of the literature. Clin Cardiol. 1991; 14:425–430.
6. Higgins R, Posner MC, Moosa HH, Staley C, Pataki KI, Mendelow H. Mesenteric infarction secondary to tumor emboli from primary aortic sarcoma. Guidelines for diagnosis and management. Cancer. 1991; 68:1622–1627.
7. Rusthoven C, Shames ML, Bui MM, Gonzalez RJ. High-grade undifferentiated pleomorphic sarcoma of the aortic arch: a case of endovascular therapy for embolic prophylaxis and review of the literature. Vasc Endovascular Surg. 2010; 44:385–391.
8. Kim KB, Kim SH. Primary undifferentiated high-grade pleomorphic sarcoma in the perihepatic space: a report of a case. J Korean Soc Radiol. 2015; 73:49–52.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download