Abstract
Epithelioid angiomyolipoma (EAML) is an uncommon, variant type of angiomyolipoma with a possibility of metastatic transformation and distant spread. Angiomyolipomas, including epithelioid variant type, are mostly renal in origin. EAML originating from extrarenal location is very rare and imaging findings are not well described. Herein, we reported a very rare case of EAML arising from the perirenal space that mimicked a malignant retroperitoneal tumor, in a 39-year-old female.
Epithelioid angiomyolipoma (EAML) is an uncommon variant type of angiomyolipoma (AML), occurring primarily in the kidney and characterized by a distinct entity with a possibility of metastatic transformation and distant spread (12).
Although the imaging findings of classical AML are well described in the literature, there are only few case reports describing the imaging appearances of EAML especially originating from the retroperitoneum (123). Herein, we reported a case of perirenal EAML with computed tomography (CT) and ultrasonography (US) findings.
A 39-year-old female with an unremarkable medical history visited the hospital via emergency department presenting with left chest pain after traffic accident. The physical examination on admission showed no abnormal finding other than tenderness at left chest wall. The patient's past medical history and family history were unremarkable. Laboratory results including blood and urine tests showed no abnormalities.
Contrast-enhanced CT of chest was performed with a 128-multidetector CT scanner (SOMATOM Definition Flash, Siemens, Muenchen, Germany). Chest CT demonstrated multiple left rib fractures with unremarkable findings in both lungs besides small amount of left pleural effusion. The CT images of the chest revealed large heterogeneous enhancing soft tissue mass in contact with the left kidney. Therefore, dynamic contrast-enhanced abdomen CT was obtained the following day, which revealed about 8.7 × 8.2 × 6.8 cm sized encapsulated soft tissue mass with abutment of the left kidney and upward displacement of the pancreas tail. Left anterior and posterior renal fascial planes were discernible around the edge of the tumor and appeared to arise from the left perirenal space. Although the tumor was in the perirenal space, normal left adrenal gland was well demonstrated (Fig. 1A).
The density of the tumor averaged about 40 Hounsfield unit (HU) at unenhanced CT images and was higher than normal renal parenchyma by about 10 HU. Even though irregular areas of low attenuation were seen in the tumor, there was no noticeable fat density (Fig. 1B). After injection of contrast material, images were obtained at 30 seconds, 80 seconds, 5 minutes, and 10 minutes. The mass showed heterogeneous enhancement with multiple, irregular internal cystic component. The pattern of dynamic enhancement revealed rapid enhancement with slow wash-out at delayed images (Fig. 1C-E). Of the solid-appearing lesions, the mean enhancement was 95, 80, 65, and 61 HU during the 30 seconds, 80 seconds, 5 minutes, and 10 minutes delayed images, respectively. Considering rapid and heterogeneous enhancement of the mass with internal irregular cystic component, initial impression included malignant retroperitoneal tumor such as sarcoma.
On US, the mass was hypoechoic compared with adjacent fat tissue and showed heterogeneous echogenicity with internal complex cystic areas. The color Doppler US of the mass revealed no appreciable internal Doppler signal. The boundary between the mass and left kidney were clearly delineated as thin hyperechoic fat echogenicity (Fig. 1F). For pathologic confirmation, US-guided biopsy of the left retroperitoneal mass was performed on patients in a prone position with a 18 gauge gun-biopsy needle.
Microscopically, the specimen was composed of dysmorphic thick-walled blood vessels, islands of mature adipose cells and predominant population of large epithelioid cells with eosinophilic cytoplasm with no evidence of mitosis or nuclear atypia (Fig. 1G). Immunohistochemical stain was strongly positive for human melanoma black (HMB)-45 and focally positive for vimentin (Fig. 1H). These findings were consistent with EAML and the patient underwent robotic surgical resection of the tumor with renal sparing. The tumor was located in the left perirenal space and was easily dissected from the surrounding tissues. There was no continuity between the tumor and left kidney. The cut surface of the specimen showed irregular pinkish hemorrhages.
After the patient was confirmed to have a retroperitoneal EAML, brain CT was evaluated in case she might have features of tuberous sclerosis but the study revealed no remarkable abnormality. The postoperative course was uneventful and after the patient was discharged, she was stable at 4 months after the operation with no sign of recurrence or metastasis on follow-up CT images.
EAML is a rare variant of AML that is characterized by positive immunoreactivity for HMB-45 and is frequently associated with tuberous sclerosis (14). While AML is almost always benign, epithelioid variant is a distinct entity which has a possibility of metastatic transformation and distant spread with relatively poor outcome (12).
EAMLs mostly occur in one or both kidneys and extrarenal EAMLs are very uncommon, with liver being the most common site (5); they also have been described in retroperitoneum, uterus, liver, lung, breast, cardiac septum, pancreas, prostate, and gastrointestinal tract (1236).
Histologically, AML is a complex mesenchymal tumor characterized by a thick walled blood vessel, smooth muscle and mature adipose tissue. However, EAML is composed of epithelioid cells with eosinophilic cytoplasm, prominent nucleoli and scarce adipose tissue, smooth muscle (13).
Due to the histologic character of EAMLs, and lack grossly visible fat on imaging, it is challenging to differentiate from other masses of retroperitoneal origin. The differential diagnosis of an enhancing mass in perirenal space may include renal cell carcinoma with perirenal spread, malignant fibrous histiocytoma, leukemia, lymphoma, metastasis, Castleman disease, leiomyoma, hemangioma, and malakoplakia (78). Because of overlap in imaging findings among the diverse perirenal lesions, a definitive diagnosis in most cases can be established on histopathologic analysis (7).
As in our case, the imaging features of EAML include hyperattenuating mass at unenhanced CT, lack of fat density, markedly heterogenous enhancement (rapid wash-in and slow wash-out), distinct margin, and frequent presence of necrotic areas (910). The high density of the EAML at unenhanced CT can be attributed to the densely packed cells, mainly in a pattern of epithelioid cells (9).
In summary, EAML of perirenal origin is an unusual entity, which is a potentially malignant tumor that has imaging appearance similar to other retroperitoneal masses. It should be considered in the differential diagnosis for a retroperitoneal mass that can occur in patients with or without tuberous sclerosis; and biopsy is recommended for a proper diagnosis, particulary in view of a possible renal-sparing treatment.
Figures and Tables
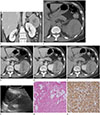
Fig. 1
Retroperitoneal epithelioid angiomyolipoma in a 39-year-old female with no associated symptom.
A. Coronal CT image reveals location of the mass in relation to the left kidney. Normal left adrenal gland (arrow) and superior displacement of the pancreas (arrowhead) are noted.
B. Unenhanced CT image shows large, high-density mass abutting to the left kidney with internal low attenuated lesions.
C-E. Mass shows rapid contrast enhancement with slow wash-out on 30 second delayed (C), 75 second delayed (D), and 5 min delayed (E) phase images.
F. US image demonstrates heterogeneous echoic mass with internal cystic areas and the mass is separated from the left kidney by thin hyperechoic fat (arrow).
G, H. Microscopically, the specimen is composed of dysmorphic thick walled blood vessels, islands of mature adipose cells and predominant population of large epithelioid cells with eosinophilic cytoplasm (G, hematoxylin and eosin staining, original magnification × 40) and epithelioid cells reveals strong and diffuse HMB-45 immunopositivity (H, HMB-45 immunohistochemical staining, original magnification × 400).
HMB = human melanoma black, US = ultrasonography
References
1. Lau SK, Marchevsky AM, McKenna RJ Jr, Luthringer DJ. Malignant monotypic epithelioid angiomyolipoma of the retroperitoneum. Int J Surg Pathol. 2003; 11:223–228.
2. Gupta C, Malani AK, Gupta V, Singh J, Ammar H. Metastatic retroperitoneal epithelioid angiomyolipoma. J Clin Pathol. 2007; 60:428–431.
3. Yokoo H, Isoda K, Nakazato Y, Nakayama Y, Suzuki Y, Nakamura T, et al. Retroperitoneal epithelioid angiomyolipoma leading to fatal outcome. Pathol Int. 2000; 50:649–654.
4. Wen J, Li HZ, Ji ZG, Mao QZ, Shi BB, Yan WG. Renal epithelioid angiomyolipoma without obvious local progress in 10 years: a case report and literature review. Ir J Med Sci. 2011; 180:557–560.
5. Ameurtesse H, Chbani L, Bennani A, Toughrai I, Beggui N, Kamaoui I, et al. Primary perivascular epithelioid cell tumor of the liver: new case report and literature review. Diagn Pathol. 2014; 9:149.
6. Kanazawa A, Fujii S, Godai TI, Ishibe A, Oshima T, Fukushima T, et al. Perivascular epithelioid cell tumor of the rectum: report of a case and review of the literature. World J Surg Oncol. 2014; 12:12.
7. Surabhi VR, Menias C, Prasad SR, Patel AH, Nagar A, Dalrymple NC. Neoplastic and non-neoplastic proliferative disorders of the perirenal space: cross-sectional imaging findings. Radiographics. 2008; 28:1005–1017.
8. Choi SY, Sung DJ, Han NY, Park BJ, Kim MJ, Sim KC, et al. Renal malakoplakia with wide extension into the retroperitoneum: a case report. J Korean Soc Radiol. 2015; 73:62–65.
9. Cui L, Zhang JG, Hu XY, Fang XM, Lerner A, Yao XJ, et al. CT imaging and histopathological features of renal epithelioid angiomyolipomas. Clin Radiol. 2012; 67:e77–e82.
10. Bharwani N, Christmas TJ, Jameson C, Moat N, Sohaib SA. Epithelioid angiomyolipoma: imaging appearances. Br J Radiol. 2009; 82:e249–e252.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download