Abstract
Intracranial hemangiopericytomas (HPC) are uncommon tumors, and their intraventricular occurrence is even rarer. Although the histopathologic findings in HPC are distinct, the diagnosis of intraventricular HPC can be difficult owing to its rarity and nonspecific clinicoradiologic manifestations. Here we present a case of intraventricular anaplastic HPC in a 20-year-old female patient, confirmed on histopathologic examination. We suggest that HPC should be considered in the differential diagnosis of space-occupying lesions of the ventricles. This article also highlights a situation in which clinical suspicion led to a meticulous radiologic review.
Hemangiopericytoma (HPC), a vascular mesenchymal neoplasm, arises from the pericytes of Zimmerman, which are leiomyoblastic cells spiraling around capillaries and postcapillary venules (12). HPC are classified by the World Health Organization (WHO) as grade II neoplasms or, if anaplastic elements are identified, grade III neoplasms. Intracranial HPC comprises 0.4% of all intracranial neoplasms and 2 to 3% of all primary tumors of the dura (3). Intraventricular locations are exceedingly rare for intracranial HPC, with only 14 case reports described in the literature (45). To the best of our knowledge, our patient is the second case of anaplastic intraventricular HPC. Here we present a case of intraventricular anaplastic HPC, along with histopathologic findings and the results of computed tomography (CT) and magnetic resonance imaging (MRI). We also review the relevant literature regarding this disease.
A 20-year-old female patient presented with headache that had lasted for 1 week prior to admission. She was sent to the emergency department for management. On the initial neurologic examination, no neurologic deficit was found, and muscle strength and deep tendon reflexes in both the upper and the lower extremities were normal. Radiologic studies were requested to evaluate any intracranial abnormality. Non-enhanced brain CT images revealed a well-circumscribed, homogeneously hyperdense mass measuring 4.1 × 3.2 cm, with peritumoral edema in the trigone of left lateral ventricle (Fig. 1). Brain MRI showed a well-circumscribed mass with heterogeneous T2 hyperintensity and T1 isointensity in the trigone of the left lateral ventricle. This tumor demonstrated homogeneous strong enhancement, with multiple intratumoral hemorrhagic foci. There was peritumoral edema in the adjacent brain parenchyma (Fig. 2). On the left vertebral angiogram, the tumor was found to be fed by branches of the left posterior choroidal artery and showed dense staining (Fig. 3).
The tumor was resected via a left parietotemporal craniotomy. During the operation, the tumor was found to be highly vascular and consisted of a soft, brownish component. Histopathologic examination confirmed that the lesion was an anaplastic HPC (WHO grade III). The specimen was composed of brownish-gray soft tissue. Microscopic analysis revealed dense cellularity with hemorrhagic foci, nuclear pleomorphism, and brisk mitotic activity. There was no evidence of microcyst formation or intratumoral necrosis. On immunohistochemical analysis, the lesion was positive for vimentin, CD34, and CD99 and focally positive for BCL-2, with a 20% Ki-67 index. Reticulin stain was positive within individual tumor cells (Fig. 4). After the surgery, the patient received adjuvant radiotherapy. On MR imaging at the 12-month follow-up visit, there was no evidence of residual or recurrent tumor.
Intracranial HPC is an unusual tumor, with an overall incidence of less than 1% among all intracranial tumors (6). These tumors show aggressive clinical behavior, with a predisposition for metastases and recurrence. HPC are classified by the WHO as grade II or, if anaplastic elements are identified, grade III neoplasms. Intraventricular locations of HPC are exceedingly rare, and only 14 case reports of intraventricular HPC have been described in the literature (45). However, to the best of our knowledge, our patient is the second case of anaplastic intraventricular HPC.
The location of intracranial HPC is similar to that of meningiomas. The most common location of HPC is the middle cranial fossa, followed by the posterior cranial fossa, the falx, and the anterior cranial fossa (7). Involvement of the skull base and dural sinus is not uncommon (8). The diagnosis of intraventricular HPC can be difficult owing to its rarity and nonspecific clinicoradiologic manifestations. Common tumors that arise from the lateral ventricle include central neurocytomas, ependymomas, subependymomas, choroid plexus papillomas, colloid cysts, and meningiomas. Among these tumors, HPC is considered to be a radiologic mimic of meningiomas. The HPC is usually manifest as a large, multilobulated tumor that, at the time of presentation, exceeds 4 cm in its greatest dimension. The typical HPC has a broad base of dural attachment but may also show a narrow dural base. Bone erosion is frequently seen. On CT images, an HPC shows hyperdense, dural-based masses with vivid enhancement. In contrast to meningiomas, HPC do not show intratumoral calcifications and adjacent bony hyperostosis (7). On conventional MR imaging, HPC have features similar to those of meningioma; however, unlike meningiomas, HPC are typically heterogeneous on imaging. HPC usually show isointensity on the T1-weighted image and heterogeneous hyperintensity on the T2-weighted image. These tumors show strong enhancement, with internal flow signal voids and peritumoral edema, and demonstrate intermediate diffusion restriction with an increase in the myoinositol peak on MR spectroscopy. On cerebral angiography, HPC show hypervascular tumor staining fed by branches of the anterior and posterior choroidal arteries (910).
In our patient, the tumor showed heterogeneous T2 hyperintensity and intratumoral hemorrhage. These findings are uncommon in the typical meningioma. Thus, initial radiologic differential diagnoses were focused on malignant intraventricular tumors in the young adult, including solitary fibrous tumor, hemorrhagic metastasis, anaplastic meningioma, and primitive neuroectodermal tumor. On histopathologic diagnosis, the most important differential diagnosis of HPC is solitary fibrous tumor, which is a non-meningothelial and mesenchymal tumor (10). As shown in our patient, positive results for CD34 and reticulin staining distinguished solitary fibrous tumor from HPC.
In clinical practice, it is important to differentiate HPC from other similar tumors because HPC are often associated with local recurrence and metastasis. The principal treatment of choice for HPC is radical or total excision regardless of the tumor location. The benefit of adjuvant radiotherapy remains controversial. Although there is no consensus protocol for the follow-up management of patients with HPC, consistent long-term imaging follow-up is recommended owing to their unpredictable and aggressive behavior (34510).
We have presented a case of intraventricular anaplastic HPC as assessed with multiple imaging modalities and histopathologic findings. Because of its rarity and nonspecific clinicoradiologic features, intraventricular HPC is often difficult to differentiate from other intraventricular tumors such as meningioma, schwannoma, and solitary fibrous tumor, etc. Therefore, we suggest that clinical awareness and suspicion are very important for accurate diagnosis and management in patients with space-occupying lesions of ventricles.
Figures and Tables
Fig. 1
Intraventricular anaplastic hemangiopericytoma in a 20-year-old woman who presented with a headache of 1 week's duration. This non-enhanced axial computed tomographic image shows a well-circumscribed hyperdense mass measuring 4.1 × 3.2 cm (arrow), with peritumoral edema in the trigone of the left lateral ventricle.
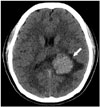
Fig. 2
MR images of intraventricular anaplastic hemangiopericytoma.
A. On the axial T1-weighted image, the tumor (arrow) shows predominant isointensity with trigonal enlargement.
B. This axial T2-weighted image reveals multiple, tiny vascular flow signal voids (arrow) within the tumor, with heterogeneous hyperintensity.
C. Fluid-attenuated inversion recovery image shows a markedly hyperintense component (arrow) in the anterior portion of the tumor.
D. On gradient echo image, countless hemorrhagic foci (arrow) can be seen in the tumor.
E. Axial gadolinium contrast-enhanced T1-weighted image shows a lobular intraventricular tumor (arrow) with strong enhancement.
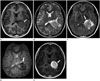
Fig. 3
Lateral left vertebral angiogram in a patient with intraventricular anaplastic hemangiopericytoma.
A. In the arterial phase, the tumor margin is delineated by feeders, including the medial posterior choroidal artery (white arrows), the lateral posterior choroidal artery (short black arrows), and splenial branches of the posterior callosal artery (long black arrows).
B. In the venous phase, the tumor shows more conspicuous staining (black double-lined arrows). There is a normal choroid plexus in front of the stained area of the tumor (white dashed arrows).
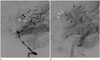
Fig. 4
Histopathologic examination of the anaplastic hemangiopericytoma (World Health Organization grade III).
A. Photomicrographs shows dense cellularity and nuclear pleomorphism with slit-like vasculature (arrows) (original magnification, × 200; hematoxylin and eosin stain).
B. Vimentin staining shows a cytoplasm-positive result (original magnification, × 200).
C. Tumor cells stained positive for reticulin, highlighting blood vessels and pericellular reticulin (original magnification, × 200).

References
1. Muller J, Mealey J Jr. The use of tissue culture in differentiation between angioblastic meningioma and hemangiopericytoma. J Neurosurg. 1971; 34:341–348.
2. Brunori A, Delitala A, Oddi G, Chiappetta F. Recent experience in the management of meningeal hemangiopericytomas. Tumori. 1997; 83:856–861.
3. Rutkowski MJ, Sughrue ME, Kane AJ, Aranda D, Mills SA, Barani IJ, et al. Predictors of mortality following treatment of intracranial hemangiopericytoma. J Neurosurg. 2010; 113:333–339.
4. Avinash KS, Thakar S, Ghosal N, Hegde AS. Anaplastic hemangiopericytoma in the frontal horn of the lateral ventricle. J Clin Neurosci. 2016; 26:147–149.
5. Towner JE, Johnson MD, Li YM. Intraventricular hemangiopericytoma: a case report and literature review. World Neurosurg. 2016; 89:728.e5–728.e10.
6. Desai K, Nadkarni T, Fattepurkar S, Goel A, Shenoy A, Chitale A, et al. Hemangiopericytoma in the trigone of the lateral ventricle--case report. Neurol Med Chir (Tokyo). 2004; 44:484–488.
7. Chiechi MV, Smirniotopoulos JG, Mena H. Intracranial hemangiopericytomas: MR and CT features. AJNR Am J Neuroradiol. 1996; 17:1365–1371.
8. Rutkowski MJ, Jian BJ, Bloch O, Chen C, Sughrue ME, Tihan T, et al. Intracranial hemangiopericytoma: clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer. 2012; 118:1628–1636.
9. Sumi K, Watanabe T, Ohta T, Fukushima T, Kano T, Yoshino A, et al. Hemangiopericytoma arising in the body of the lateral ventricle. Acta Neurochir (Wien). 2010; 152:145–149. discussion 150.
10. Tanaka T, Kato N, Arai T, Hasegawa Y, Abe T. Hemangiopericytoma in the trigone of the lateral ventricle. Neurol Med Chir (Tokyo). 2011; 51:378–382.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download