Abstract
Air-leakage syndrome associated with graft-versus-host disease (GVHD) is a rare complication, but it is also reported as an independent predictor of a worse survival rate after stem cell transplantation. We report two cases of air-leakage syndrome associated with GVHD after allogeneic stem cell transplantation in acute leukemia patients who presented with spontaneous pneumomediastinum and subcutaneous emphysema, and finally death due to respiratory failure seven to eight months later.
The recent development of innovative therapeutic techniques has improved the survival rate of allogeneic stem cell transplantation (Allo-SCT) recipients with hematologic malignancies; however, noninfectious pulmonary complications as a result of chronic graft-versus-host disease (GVHD) still remain a frequent cause of transplant-related death (12). Among the late-onset noninfectious pulmonary complications associated with chronic GVHD, thoracic air-leakage syndrome (ALS), which worsens the survival rate, has been addressed in several reports of patients with bronchiolitis obliterans (BO) (3). Although ALS manifesting as pneumomediastinum, subcutaneous emphysema, pneumothorax, and pneumopericardium is a rare complication, its clinical course and cause of poor prognosis have not been well understood. To the best of our knowledge, there have only been a few reports regarding ALS in Allo-SCT recipients. Herein, we present two cases of ALS with BO in chronic GVHD patients after Allo-SCT and report their clinical course, computed tomography (CT) findings, and finally death.
A 20-year-old man received Allo-SCT for acute myeloid leukemia in August 2012. Allo-SCT was successful with good engraftment and the patient had no specific symptoms representing acute rejection. Post-transplant GVHD prophylaxis was performed with tacrolimus. His pulmonary function tests (PFT) prior to bone marrow transplantation were normal, as indicated by forced expiratory volume in 1 (FEV1)/the forced vital capacity (FVC) = 0.94, FEV1 = 4.69 L (130% predicted), and FVC = 4.97 L (127% predicted). About one year after Allo-SCT, he presented with dyspnea, general weakness, and erythema with erosions on the hard palate and lips. On chest CT during admission, consolidation mixed with ground-glass opacity lesions along the bronchovascular bundles in both sides of the lungs were thought to be a manifestation of bronchiolitis obliterans organizing pneumonia (BOOP); however, he had no fever, and there was no clinical or laboratorial evidence of infection. He had been diagnosed with chronic GVHD-extensive (involving the eyes, mouth, liver, and lung-BOOP). After treatment with high-dose glucocorticoids, the symptoms of chronic GVHD were resolved, enabling his discharge. Six months later, in March 2014, he was hospitalized due to aggravation of dyspnea, and during hospitalization, he complained of sudden chest pain which was thought to be an aggravation of noninfectious pulmonary complications of chronic GVHD. Crepitus was heard over the anterior chest wall and lower neck on physical examination, and PFTs showed a mixed pattern (severe obstruction of the small airways and a restrictive pattern), as indicated by FEV1/FVC = 0.36, FEV1 = 1.08 L (25% predicted), and FVC = 3.04 L (59% predicted). Chest radiograph showed pneumomediastinum and subcutaneous emphysema (Fig. 1A). Chest CT scan demonstrated extensive subcutaneous emphysema along both sides of the neck and chest wall, pneumomediastinum extending to the upper, middle, and posterior mediastinum, and pulmonary interstitial emphysema (Fig. 1B-D). Not only multifocal patchy ground-glass opacities along the bronchovascular bundles, but also newly developed bronchiectasis and a mosaic pattern of lung attenuation mixed with paucity of pulmonary vasculature suggesting BO were noted. Because there was no history of trauma and no predisposing factor for pneumomediastinum, in spite of normal esophagography, he was finally diagnosed with thoracic ALS as a late-onset noninfectious pulmonary complication associated with chronic GVHD in an Allo-SCT recipient. The patient was treated with steroid pulse therapy and he underwent conservative treatment including high-flow oxygen and bronchodilators. Subcutaneous emphysema and pneumomediastinum resolved completely, and he was discharged after one month of treatment. However, 8 months after discharge, he presented with severe dyspnea and subsequent PFTs showed worsened findings as indicated by FEV1/FVC = 0.23, FEV1 = 0.53 L (12% predicted), and FVC = 2.28 L (44% predicted). Despite intensive treatment for 2 months after hospitalization, he died due to respiratory failure associated with aggravation of GVHD, approximately 27 months after Allo-SCT.
The following is another case of thoracic air-leakage syndrome (ALS) associated with GVHD. A 58-year-old man received allogeneic peripheral blood stem cell transplantation for the treatment of acute lymphoid leukemia in November 2009. Allo-SCT was successful, with good engraftment, and the patient had no specific symptoms representing acute rejection. Post-transplant GVHD prophylaxis was performed with tacrolimus. He was doing well, but approximately three years later he presented with cough, sputum, and dyspnea. He underwent chest CT, and tubular bronchiectasis was detected with bronchial wall thickening in the lobar and segmental bronchi in all five lobes, and a mosaic pattern of lung attenuation suggesting BO was also noted (Fig. 2A). PFTs showed a severe obstructive pattern of the small airways, as indicated by FEV1/FVC = 0.26, FEV1 = 0.87 L (29% predicted), and FVC = 3.38 L (82% predicted). He had no fever, and there was no clinical and laboratorial evidence of infection. Clinically, he had been diagnosed with BO as a manifestation of noninfectious pulmonary complications of chronic GVHD and treated with high-dose steroids. One and a half years later, in April 2014, he experienced sudden chest and neck pain, and crepitus could be heard over the neck and anterior chest wall on physical examination. The subsequent PFTs showed worsened findings due to severe obstruction, as indicated by FEV1/FVC = 0.22, FEV1 = 0.68 L (23% predicted), and FVC = 3.06 L (75% predicted). Chest radiograph demonstrated subcutaneous emphysema and pneumomediastinum as well as hyperinflation (increased radiolucency) of both sides of the lungs. On chest CT, newly developed pneumomediastinum and subcutaneous emphysema were noted in the lower neck and upper thoracic cage and progression of bronchiectasis in the underlying BO was also noted on follow up chest CT images (Fig. 2B, C). No causes of air leakage were found in the thorax, and he was diagnosed with thoracic ALS as a late-onset noninfectious pulmonary complication associated with chronic GVHD in an Allo-SCT recipient. The patient was treated with steroid pulse therapy and conservative treatments, including high-flow oxygen and bronchodilators. Subcutaneous emphysema and pneumomediastinum completely resolved, and he was discharged after one month of careful treatment. However, seven months later, he presented with sudden, severe dyspnea. Despite intensive treatment for 3 weeks after hospitalization, he died due to respiratory failure associated with aggravation of GVHD approximately 62 months after Allo-SCT.
In these rare cases, ALS occurred in the underlying noninfectious pulmonary complications of BO, and even with complete resolution of thoracic air leakage, both patients died within several months and their deaths were attributed to respiratory failure associated with complications of chronic GVHD in Allo-SCT recipients.
Chronic GVHD is the most common complication, occurring in approximately 60% to 80% of long-term survivors of Allo-SCT. There are several reports regarding late-onset noninfectious pulmonary complications including BO/BOOP associated with GVHD, and most patients with these complications have responded to proper treatment. However, concomitant ALS after stem cell transplantation is a very rare complication and radiologists are not very familiar with this condition (456). Franquet et al. (6) defined ALS as the presence of extra-alveolar air, including spontaneous pneumomediastinum, pneumopericardium, subcutaneous emphysema, interstitial emphysema, and spontaneous pneumothorax attributed mainly to BO/BOOP associated with chronic GVHD. BO/BOOP is known for being the most common cause of ALS, and ALS can be an initial manifestation of BO/BOOP (37). Although the occurrence of BO/BOOP as a complication of chronic GVHD has been reported in 2% to 14% of Allo-SCT recipients who survive for more than 3 months, the incidence of ALS in Allo-SCT recipients with chronic GVHD-BO/BOOP is between 0.8% to 2.3%, but this is still unclear due to the scarcity of reports (6789). Although our cases were not pathologically confirmed as BO, clinically definite obstructive symptoms with the evidence of severe obstruction in PFTs as well as radiologic findings of bronchiectasis, a mosaic pattern of lung attenuation, and paucity of pulmonary vasculature could be strong evidence for the diagnosis of BO.
Thoracic air leakage in subcutaneous emphysema and pneumomediastinum may occur in the underlying conditions of asthma, forced Valsalva maneuvers, or occult pneumothorax. However, these possible predisposing factors were ruled out in our cases. The known pathogenesis of ALS is as follows: alveolar rupture causes interstitial emphysema, as seen in case 1 presented in this paper, which then dissects along the bronchovascular bundle sheaths to the pulmonary hilum and into the mediastinum, otherwise called the "Macklin effect" (10). Alveolar rupture may occur in the presence of increased intra-alveolar pressure in case of coughing or vomiting or a damaged alveolar wall in cases of noninfectious (emphysema, bulla, BO) or pulmonary infection.
According to previous reports, after receiving treatment according to the type of ALS, the response was poor and the mortality rate of ALS was 66.7-100% (78). ALS could not be easily treated by regular means of treating pneumomediastinum, subcutaneous emphysema, or pneumothorax, and it can be regarded as an indicator of poor prognosis in Allo-SCT recipients with chronic GVHD-BO/BOOP. Although our cases demonstrate that thoracic air leakage had completely resolved in both patients after one month of proper treatment, 7 to 8 months later both patients expired due to acute respiratory failure.
Several risk factors for ALS have been identified in Allo-SCT recipients (3): chronic GVHD, second or subsequent SCT, male sex, an age of less than 38 years, and tacrolimus-based GVHD prophylaxis. Furthermore, ALS itself was also identified as an independent predictor of worse survival after Allo-SCT. In our cases, chronic GVHD, male sex, an age of 20 years in the first case, and tacrolimus-based GVHD prophylaxis were identified as known risk factors for ALS, and both cases resulted in death.
In summary, we present two cases of ALS in Allo-SCT recipients with chronic GVHD-BO. Although ALS is very rare and seems to be an incidental and unfamiliar complication after SCT, it is in fact associated with a worse survival rate and high mortality in Allo-SCT recipients. In practice, radiologists should be consider thoracic air leakage during the regular follow-up chest CT scans of Allo-SCT recipients.
Figures and Tables
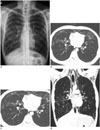 | Fig. 1A 20-year-old man underwent related allogeneic stem cell transplantation (Allo-SCT) for acute myeloid leukemia.
A. One and a half years after Allo-SCT, a plain chest radiograph shows subcutaneous emphysema in the neck and upper trunk and pneumomediastinum. Patchy ground-glass opacities (GGO) along the bronchovascular bundles are also noted in both lungs.
B-D. Chest CT scans show extensive subcutaneous emphysema in the neck and chest wall, pneumomediastinum and pneumorachis. Multifocal patchy GGOs, bronchiectasis, as well as a mosaic pattern of attenuations mixed with paucity of regional pulmonary vasculature in both lower lobes suggest a bronchiolitis obliterans component. Radiolucent air around the segmental pulmonary artery and bronchus in the right lower lobe, suggesting pulmonary interstitial emphysema (arrows in C) is also noted.
CT = computed tomography
|
 | Fig. 2A 58-year-old man underwent related allogeneic stem cell transplantation (Allo-SCT) for acute lymphoid leukemia.
A. Approximately three years after Allo-SCT, bilateral diffuse tubular bronchiectasis mixed with a mosaic pattern of lung attenuation and paucity of pulmonary vessels in the radiolucency area (air trapping) suggesting bronchiolitis obliterans are noted in both lower lobes on chest CT.
B, C. One and a half years later, newly developed pneumomediastinum and subcutaneous emphysema are noted in the lower neck and the upper thoracic cage. Bilateral diffuse tubular bronchiectasis and radiolucency of air trapping suggesting bronchiolitis obliterans are still noted in both sides of the lungs. Localized cystic bronchiectasis is also noted in the anterior segment of the right upper lobe.
CT = computed tomography
|
References
1. Shin HJ, Park CY, Park YH, Kim YJ, Min CK, Lee S, et al. Spontaneous pneumothorax developed in patients with bronchiolitis obliterans after unrelated hematopoietic stem cell transplantation: case report and review of the literature. Int J Hematol. 2004; 79:298–302.
2. Soubani AO, Uberti JP. Bronchiolitis obliterans following haematopoietic stem cell transplantation. Eur Respir J. 2007; 29:1007–1019.
3. Sakai R, Kanamori H, Nakaseko C, Yoshiba F, Fujimaki K, Sakura T, et al. Air-leak syndrome following allo-SCT in adult patients: report from the Kanto Study Group for Cell Therapy in Japan. Bone Marrow Transplant. 2011; 46:379–384.
4. Hildebrandt GC, Fazekas T, Lawitschka A, Bertz H, Greinix H, Halter J, et al. Diagnosis and treatment of pulmonary chronic GVHD: report from the consensus conference on clinical practice in chronic GVHD. Bone Marrow Transplant. 2011; 46:1283–1295.
5. Afessa B, Litzow MR, Tefferi A. Bronchiolitis obliterans and other late onset non-infectious pulmonary complications in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001; 28:425–434.
6. Franquet T, Rodríguez S, Hernández JM, Martino R, Giménez A, Hidalgo A, et al. Air-leak syndromes in hematopoietic stem cell transplant recipients with chronic GVHD: high-resolution CT findings. J Thorac Imaging. 2007; 22:335–340.
7. Moon MH, Sa YJ, Cho KD, Jo KH, Lee SH, Sim SB. Thoracic air-leak syndromes in hematopoietic stem cell transplant recipients with graft-versus-host disease: a possible sign for poor response to treatment and poor prognosis. J Korean Med Sci. 2010; 25:658–662.
8. Vogel M, Brodoefel H, Bethge W, Faul C, Hartmann J, Schimmel H, et al. Spontaneous thoracic air-leakage syndrome in patients following allogeneic hematopoietic stem cell transplantation: causes, CT-follow up and patient outcome. Eur J Radiol. 2006; 60:392–397.
9. Toubai T, Tanaka J, Kobayashi N, Honda T, Miura Y, Ogawa T, et al. Mediastinal emphysema and bilateral pneumothoraces with chronic GVHD in patients after allogeneic stem cell transplantation. Bone Marrow Transplant. 2004; 33:1159–1163.
10. Wintermark M, Schnyder P. The Macklin effect: a frequent etiology for pneumomediastinum in severe blunt chest trauma. Chest. 2001; 120:543–547.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download