Abstract
Glioblastoma multiforme (GBM) most commonly occurs in the pons while it is rare in the brainstem. However, diagnosis of brainstem GBM can be difficult due to its rarity and nonspecific clinical manifestations. Herein, we presented a case of a 47-year-old female patient confirmed as primary pontine GBM by histopathological examination. This case highlights that GBM should be considered in the differential diagnosis of patients with a space-occupying lesion in the brainstem as well as the importance of a meticulous radiological review with clinical suspicion.
Brainstem gliomas are rare in adults and represent < 2% of gliomas. A small number of these gliomas are glioblastoma multiformes (GBMs) (1). In contrast to pediatric patients, adult brainstem gliomas show heterogeneous natures and have variable radiologic findings and prognoses. Few reports describe brainstem GBMs with radiologic-pathologic correlation due to the overall rarity of brainstem GBMs and the limitations of tissue confirmation caused by procedure-related risks (2). Herein, we presented a case of primary pontine GBM with computed tomography (CT), magnetic resonance imaging (MRI), and histopathologic findings. We also performed a relevant literature review on this disease.
A 47-year-old female patient presented with drowsy mental state and gait disturbance for 30 days before admission. At the initial neurological examination, there was neurologic deficit of the left cranial nerve VI, VII, and VIII with horizontal diplopia, hemifacial palsy, absent corneal reflex, and hearing disturbance. There were no abnormal findings for muscle strength and deep tendon reflexes in both the upper and lower extremities. Radiological evaluations were requested for suspicion of a space occupying lesion during admission. Contrast enhanced CT images revealed a poorly defined, isoattenuated mass-like lesion in the pons and left cerebellopontine angle. This lesion showed heterogeneous enhancement with a central non-enhancing component and measured 3.2 × 2.6 cm (Fig. 1). MRI showed an infiltrative mass with an indistinct margin in the pons and left cerebellopontine cistern, which extended to the left Meckel's cave. This tumor was homogenously hypointense on T1-weighted images and heterogeneously hyperintense on T2-weighted images. The left superior cerebellar artery was encased by the tumor. The lesion showed a heterogeneous enhancement with non-enhancing component following injection of gadolinium, which suggested intratumoral necrosis (Fig. 2). Peritumoral edema was observed in the adjacent cerebellum and ipsilateral midbrain. The patient underwent a left suboccipital craniotomy for stereotactic biopsy. The histopathological examination confirmed that the tumor was a GBM. The specimen was composed of yellowish-gray and pinkish-gray soft tissues. The microscopic analysis showed a dense cellularity and ill-defined cell borders with extensive cytoplasmic and numerous nuclear pleomorphism. The immunohistochemistry analysis revealed that the lesion was positive for glial fibrillary acidic protein with a 40% Ki-67 index and 3% p53 index (Fig. 3). After the surgery, the patient received concomitant chemo-radiotherapy.
Brainstem GBMs are extremely rare in adults, and the majority of these tumors occur in the pons (1). Badhe et al. (3) performed a retrospective analysis of 45 cases of brainstem gliomas and found that 24% occurred in patients > 20 years of age. Additionally, 15% percent of tumors were grade IV. Most of the tumors were located in the pons (55%), followed by the medulla (31%), and the midbrain (13%). However, a previous study of 100 histologically confirmed adult brainstem gliomas revealed that anaplastic astrocytomas (43%) were the most common glioma followed by GBMs (28%) and diffuse astrocytomas (15%) (4).
The clinical features are variable depending on the tumor location. The symptoms include the following: cranial nerve palsy, cerebellar sign, visual disturbance, headache, sensory disturbance, pyramidal sign, gait disturbance, motor weakness, or sensory change (5). The diagnosis and classification of brainstem GBMs are often based on radiological findings, especially by MRI because of the overall rarity and limited available tissue (246). CT findings of brainstem GBMs typically show a hypodense or isodense mass (7). The MRI findings usually appear as a heterogeneously enhancing infiltrative mass with T1 hypointensity and T2 hyperintensity (789). GBMs may also present as a heterogeneous signal intense mass. The tumor often reveals non-enhancing intratumoral components that suggest necrosis and surrounding edema. Kwon et al. (10) demonstrated that rapid diffusion MRI, thallium single photon emission CT and positron emission tomography could increase the diagnostic yield. These radiological findings are the most important for initial evaluation of the brainstem GBM. However, it is often difficult to differentiate brainstem GBM from other diseases, which may have similar imaging features such as non-glial high grade tumors, infectious, inflammatory, autoimmune or vascular diseases in clinical practice (25). Therefore, a surgical biopsy is used to make a definite diagnosis of the brainstem lesion. Another advantage of surgical biopsy is tumor decompression, which leads to better therapeutic outcomes. Although image-guided stereotactic biopsy of the brainstem is considered a safe and reliable procedure, the optimal methods and routes of biopsies are still debated (25).
Herein, we reported a case of primary pontine GBM in a patient with multiple neurologic deficits. The radiological and clinical findings of the brainstem lesion can be nonspecific as shown our case, and the rarity of brainstem GBM in adults can lead to an even more challenging diagnosis in clinical practice. Although it is often difficult to differentiate GBM from other mimicking diseases, GBM should be considered for a radiologic differential diagnosis in patients with space-occupying brainstem lesions.
Figures and Tables
Fig. 1
Representative MRI of primary pontine glioblastoma multiforme in a 47-year-old female patient.
A. Axial T2-weighted MR image demonstrates a heterogenously hyperintense tumor with partially defined margin.
B. Axial pre-contrast T1-weighted MR image reveals a homogeneously hypointense tumor.
C. Axial post-contrast fluid attenuated inversion recovery image shows a heterogeneously enhancing tumor with partial obstruction of the fourth ventricle and peritumoral vasogenic edema.
Fig. 2
Representative MRI of primary pontine glioblastoma multiforme in a 47-year-old female patient.
A. Axial T2-weighted MR image demonstrates a heterogenously hyperintense tumor with partially defined margin.
B. Axial pre-contrast T1-weighted MR image reveals a homogeneously hypointense tumor.
C. Axial post-contrast fluid attenuated inversion recovery image shows a heterogeneously enhancing tumor with partial obstruction of the fourth ventricle and peritumoral vasogenic edema.
D-F. Axial (D), sagittal (E), and coronal (F) enhanced T1-weighted MR images show a heterogeneously enhancing tumor with intratumoral necrosis involving pons and left Meckel's cave.
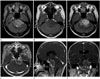
Fig. 3
Pathologic findings of primary pontine glioblastoma multiforme in a 47-year-old female patient.
A. Photomicrograph (original magnification, × 100; hematoxylin and eosin stain) shows dense cellularity, nuclear pleomorphism and a variable nuclear:cytoplasmic ratio.
B. GFAP staining (immunohistochemistry, × 200) shows diffusely positive immunoreactivity, presenting brownish staining. This result indicates astrocytic nature of tumor.
C. Approximately 40% of cells have Ki-67 reactivity, which demonstrates increased cell proliferation (original magnification, × 200; Ki-67 stain). GFAP = glial fibrillary acidic protein

References
1. Guillamo JS, Monjour A, Taillandier L, Devaux B, Varlet P, Haie-Meder C, et al. Brainstem gliomas in adults: prognostic factors and classification. Brain. 2001; 124(Pt 12):2528–2539.
2. Rachinger W, Grau S, Holtmannspötter M, Herms J, Tonn JC, Kreth FW. Serial stereotactic biopsy of brainstem lesions in adults improves diagnostic accuracy compared with MRI only. J Neurol Neurosurg Psychiatry. 2009; 80:1134–1139.
3. Badhe PB, Chauhan PP, Mehta NK. Brainstem gliomas--a clinicopathological study of 45 cases with p53 immunohistochemistry. Indian J Cancer. 2004; 41:170–174.
4. Theeler BJ, Ellezam B, Melguizo-Gavilanes I, de Groot JF, Mahajan A, Aldape KD, et al. Adult brainstem gliomas: correlation of clinical and molecular features. J Neurol Sci. 2015; 353:92–97.
5. Reyes-Botero G, Mokhtari K, Martin-Duverneuil N, Delattre JY, Laigle-Donadey F. Adult brainstem gliomas. Oncologist. 2012; 17:388–397.
6. Fischbein NJ, Prados MD, Wara W, Russo C, Edwards MS, Barkovich AJ. Radiologic classification of brain stem tumors: correlation of magnetic resonance imaging appearance with clinical outcome. Pediatr Neurosurg. 1996; 24:9–23.
7. Lakhan SE, Harle L. Difficult diagnosis of brainstem glioblastoma multiforme in a woman: a case report and review of the literature. J Med Case Rep. 2009; 3:87.
8. Kuroiwa T, Numaguchi Y, Rothman MI, Zoarski GH, Morikawa M, Zagardo MT, et al. Posterior fossa glioblastoma multiforme: MR findings. AJNR Am J Neuroradiol. 1995; 16:583–589.
9. Zhang Y, Pan Y, Li X. Glioblastoma multiforme in the brainstem in a young adult. Clin Neurol Neurosurg. 2014; 124:175–178.
10. Kwon JW, Kim IO, Cheon JE, Kim WS, Moon SG, Kim TJ, et al. Paediatric brain-stem gliomas: MRI, FDG-PET and histological grading correlation. Pediatr Radiol. 2006; 36:959–964.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download