INTRODUCTION
The greater omentum is frequently involved in the intra-abdominal dissemination of gastrointestinal and ovarian malignancies, either during primary seeding or as a site of recurrent cancer after surgical treatment (1234). Therefore, the greater omentum, historically, was totally resected during surgery for abdominal cancer. However, several studies of omentum-preserving operations show a lower rate of postoperative complications and no statistically significant difference in patient survival, as compared with total omentectomy (567). Therefore, the use of omentum-preserving operations has recently been increasing, especially for gastric cancer surgery. However, a variety of benign lesions of traumatic, inflammatory, vascular, or systemic origin can occur in the remnant omentum. Various findings are visible on computed tomography (CT), including hazy stranding, nodules, mass formation, and diffuse infiltration.
In this article, we review the CT findings of a variety of benign omental lesions after abdominal cancer surgery. The appearances of benign omental lesions on CT are classified into four categories, according to the etiopathogenic mechanism: 1) traumatic omental lesions; 2) inflammatory omental lesions; 3) vascular omental lesions; and 4) omental lesion from systemic causes.
ANATOMY
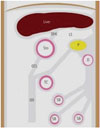
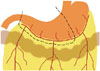
The greater omentum is the largest peritoneal fold and is composed of descending and ascending portions that fuse to form a four-layer vascular fatty apron (89). The greater omentum extends from the greater curvature of stomach, covers the small bowel, and then folds back to fuse with the transverse colon and transverse mesocolon (Fig. 1). Blood is supplied to the greater omentum from the right and left gastroepiploic arteries, which branch into three vessels: right, left, and middle omental arteries (10).
On CT, the greater omentum normally appears as a band of fatty tissue with a variable width, located just beneath the anterior abdominal wall and anterior to the stomach, transverse colon, and small bowel.
TRAUMATIC OMENTAL LESION
Omental Contusion
Postoperative omental contusion is reportedly related to intraoperative mechanisms, ranging from retraction injury to omental manipulation (11). Omental contusion is a temporary and self-limiting post-surgical change.
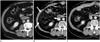
On CT, the linear soft tissue density stranding and higher attenuation of omental fat can be seen in the early postoperative period (Fig. 2). These findings correspond to a contusive edema or hemorrhagic infiltration of the omentum (12).
On short-term follow-up CT, complete resolution of these findings can be favorable for diagnosis of omental contusion. Persistence of omental lesions on follow-up CT can reliably exclude the diagnosis of omental contusion.
Omental Hematoma
Omental hematoma usually occurs during penetrating or blunt trauma, including seat belt-related injuries in high-speed deceleration events. It is caused by rupture or dissection of the omental vasculature (1314). Omental hematoma can also occur as a postoperative condition by a similar mechanism. In laparoscopic surgery, omental hematoma can occur near the trocar port site, as a result of direct injury to the omental vasculature. Additionally, it might also be related with failed surgical hemostasis of the omentum, such as slippage of the hemostatic suture or clip.
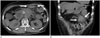
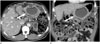
The CT findings of omental hematoma are similar to those of hemorrhage in other sites. During active bleeding, contrast-enhanced CT shows extravasation of contrast material, and nonenhanced CT shows a high-attenuated (40–60 Hounsfield unit), mass-like lesion in the omentum (Fig. 3). The diagnosis of omental hematoma is facilitated by locating the hematoma near the trocar site of laparoscopic surgery. The follow-up CT reveals gradual resolution of the hematoma.
Heterotopic Omental Ossification
Heterotopic ossification is abnormal bone formation in tissues that do not normally ossify. Most intra-abdominal heterotopic ossification occurs in the mesentery, which is called heterotopic mesenteric ossification. Omental involvement of heterotopic ossification is an extremely rare event that is called heterotopic omental ossification and considered one type of heterotopic mesenteric ossification (15).
The pathogenesis remains unknown, but is generally accepted as the differentiation of immature multipotent mesenchymal cells into osteoblasts or chondroblasts as a reaction to a variety of stimuli, including trauma, burns, prolonged immobilization, infection, prior surgery, and neoplasia (1617). Heterotopic omental ossification is usually associated with laparotomy and colectomy (18). Ossification rapidly (mean, 1–2 weeks) develops postoperatively (15).
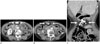
CT helps to confirm the diagnosis and shows ossification in the omentum, especially along the incision site (Fig. 4). The ossification in the omentum and mesentery can occasionally be extensive. Bone scanning with Tc 99m pyrophosphate is useful for early diagnosis before calcification or ossification is observed on a plain radiograph (1719).
INFLAMMATORY OMENTAL LESION
Omental Abscess
Postoperative omental abscess is usually associated with peritonitis caused by contamination or bowel spillage during surgery or by leakage from the stump or anastomosis site in the postoperative period (20). Omental infarction can also progress to omental abscess (1121).
The CT findings of omental abscess are similar to those of an abscess in other sites. CT reveals a well-defined fluid-density lesion with a peripheral enhanced wall (Fig. 5). Additionally, an inflammatory infiltrative process surrounding the omental abscess is seen as omental haziness and soft tissue stranding in the greater omentum. Occasionally, the abscess can demonstrate gas bubbles or air-fluid levels in gas-forming infections.
Foreign Body Granuloma (Gossypiboma)
Foreign body granuloma develops from retention of surgical foreign bodies that are left behind in the body cavity during surgery. Various surgical foreign bodies can cause foreign body granuloma. Of these, laparotomy sponges are the most commonly reported surgically retained foreign body, or gossypiboma (22). Although they can be present in any cavity, gossypibomas are most frequently discovered in the intra-abdominal cavity (23). Omental involvement is rare.
Pathologically, inert foreign bodies, such as surgical sponges, can cause two types of foreign body reaction (24). One is an aseptic fibrous response that leads to adhesions and encapsulation, resulting in granuloma formation. Another is an exudative response that induces abscess formation with or without secondary bacterial invasion, which results in various fistulas (2324). The clinical presentations of gossypiboma are broad, ranging from none to fatal, and can manifest from the immediate postoperative period to decades after surgery (232526).
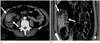
CT is the imaging modality of choice for gossypiboma. The most characteristic CT finding is a whorl-like spongiform heterogeneous hypodense mass containing air bubbles and a thick peripheral high-density wall that is further highlighted on contrast-enhanced imaging (Fig. 6) (2324). The mass might contain wavy, striped, high-density areas that represent the sponge itself (27). A long-lasting gossypiboma shows a calcified reticulate rind sign that may be ascribed to long-standing deposition of calcification along the network architecture of a surgical swab (28).
Omental Panniculitis
Intra-abdominal panniculitis is a rare idiopathic disease that is characterized by fat necrosis, chronic inflammation, and fibrosis of intra-abdominal adipose tissue. Although there is no definite cause of intra-abdominal panniculitis, it is often associated with many medical conditions and diseases, such as abdominal surgery, trauma, drugs, infection, autoimmune disease, or malignancy (29). Of these, malignancy and abdominal surgery are suggested to trigger the development of intra-abdominal panniculitis (2930).
Intra-abdominal panniculitis mainly involves the small bowel mesentery, especially at its root, and occasionally the mesocolon. It rarely involves the peripancreatic area, retroperitoneum, pelvis, and omentum (29). In particular, isolated omental involvement is extremely rare. Currently, only 5 cases of isolated omental panniculitis are reported in the literature (3132333435).
The characteristic CT findings of intra-abdominal panniculitis are widely known: slightly increased fat density with a regional mass effect, soft tissue nodules, fat-ring sign (fat density preservation around vessels and nodules), and tumoral pseudocapsule (hyperattenuating pseudocapsule surrounding fat) (2931). The fat-ring sign and tumoral pseudocapsule are very specific for the diagnosis of intra-abdominal panniculitis (293637). Isolated omental panniculitis shows varying CT features, ranging from mild infiltration to a soft tissue density mass (Fig. 7). CT can also reveal a fat-ring sign and tumoral pseudocapsule, similar to features of intra-abdominal panniculitis in other sites (3132333435).
Pancreatitis-Related Omental Lesion
The incidence of acute postoperative pancreatitis is relatively lower than other well-recognized postoperative complications. However, it is associated with a high mortality rate (38). Acute postoperative pancreatitis is mainly related with surgical injury of the pancreas that can occur during gastrectomy, such as stomach adhesion to the pancreas and pancreatic parenchymal injury from electrocauterization, traction, compromised microcirculation, and direct injury to the pancreatic duct (3940). In addition, it is related to extended lymph node dissection and distal pancreatectomy or splenectomy in cases of invasion by gastric carcinoma (3941).
Omental involvement in pancreatitis is rare. It can occur when extravasated pancreatic enzymes dissect the transverse mesocolon and spread along the omentum, which are characteristic sites involved in severe pancreatitis (942).
CT findings of postoperative pancreatitis-related omental lesion include underlying acute pancreatitis, such as segmental or diffuse enlargement of the pancreas, and extension of inflammatory infiltration, and fluid collections into the transverse mesocolon and omentum (Fig. 8). In the Atlanta classification, these omental fluid collections are classified into four types according to necrosis and elapsed time: 1) acute peripancreatic fluid collection (within 4 weeks, without necrosis); 2) pseudocyst (encapsulated collection after 4 weeks, without necrosis); 3) acute necrotic collection (within 4 weeks); and 4) walled-off necrosis (encapsulated collection after 4 weeks, with necrosis) (43). It is important to localize the origin of the inflammatory process to distinguish between secondary inflammatory changes and primary omental diseases (44).
VASCULAR OMENTAL LESION
Omental Infarction
The greater omentum is a vascular-rich structure that has numerous small vessels with abundant collaterals. Therefore, omental infarction is generally uncommon. However, compromised omental vascularization from various causes can induce segmental omental infarction, which clinically manifests as localized abdominal pain, nausea, vomiting, anorexia, diarrhea, and fever. According to the classification proposed by Leitner et al. (45), omental infarction might be primary or secondary and occur with or without torsion. Primary omental infarction, with or without torsion, is related with congenital anatomic variations, mostly in obese patients (454647). Secondary omental infarction with torsion might be caused by pathological foci of omentum such as cysts, inflammation, masses, or scars (4748). Omental infarction without torsion might be secondary to a predisposing condition, such as cardiovascular disease, trauma, heavy meals, and surgery (11).
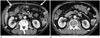
Postoperative omental infarction can be caused by torsion due to adhesion or a surgical scar and by direct cutting of omental vessels during the operation (495051). In an omentum-preserving operation, the greater omentum is partially divided, leaving the distal omentum attached to the transverse colon. The right and middle omental arteries, including the gastroepiploic artery, are cut while the omentum is partially divided (Fig. 9). Therefore, although the omentum is a vascular-rich structure, infarction can occur in the omentum during the postoperative follow-up period (52).
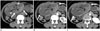
The CT findings of omental infarction progress from an ill-defined, heterogeneous fat density lesion in the early stage (< 15 days) to a well-defined, smaller lesion with a hyperdense rim (> 30 days) (47). The appearance of postoperative omental infarction on CT can be classified into four types: type 1 (ill-defined, heterogeneous, fat density lesion); type 2 (well-defined fat density lesion with rim enhancement); type 3 (well-defined heterogeneous lesion with a fat component); and type 4 (well-defined heterogeneous lesion without a fat component) (52). The initial CT appearance of postoperative omental infarction presents as a type 1 lesion, which can progress to types 2 and 3 (Fig. 10). All infarctions become smaller and better defined with evolution on follow-up CT.
Omental infarction may not contain fat components in the late stage (type 4). Therefore, the radiological differentiation between a type 4 omental infarction and omental metastasis can be difficult. In such a case, review of the previous CT images can be helpful in differentiating omental infarction from omental metastasis (52).
OMENTAL LESION BY SYSTEMIC CAUSES
Omental Edema Related with a Systemic Condition
Postoperative omental edema can be related to a preoperative systemic condition including liver cirrhosis with portal hypertension, hypoalbuminemia, nephrosis, heart failure, tricuspid disease, and constrictive pericarditis (42). Of these, liver cirrhosis with portal hypertension is one of the most common causes of omental edema (5354). When omental edema is secondary to a systemic disease, it is usually combined with mesenteric, retroperitoneal, generalized subcutaneous edema and ascites (42). In patients with liver cirrhosis, omental edema occurs in combination with mesenteric edema, and neither omental nor retroperitoneal edema occurs in the absence of mesenteric edema (55).
The CT findings of omental edema are similar to those of mesenteric edema. They vary from a mild, focal, and infiltrative haze to more diffuse and mass-like appearances, similar to features on CT of other pathologic conditions of the omentum (Fig. 11) (5355). With severe systemic conditions that cause omental edema, ascites, subcutaneous edema, and pleural effusion can also occur (4255).
On follow-up CT, complete resolution of the omental edema can be observed with improvement in the underlying systemic condition.
CONCLUSION
A variety of benign lesions of the greater omentum can be observed in patients who undergo surgery for an intra-abdominal malignancy. These can be traumatic (contusion, hematoma, heterotopic omental ossification), inflammatory (abscess, foreign body granuloma, panniculitis, omental lesion associated with pancreatitis), vascular (infarction), or systemic in origin. Varying CT findings of these lesions can be challenging for radiologists on follow-up CT. An awareness of the CT features and evolutional changes in these benign omental lesions can help ensure a correct diagnosis and avoid a misdiagnosis.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download