Abstract
Retroperitoneal liposarcomas usually present as painless, slow-growing abdominal masses. When masses grow large enough to compress surrounding structures, symptoms may occur. Retroperitoneal liposarcoma clinically manifesting as inguinal hernia is a very rare entity; only 11 cases have been reported. Herein, we present radiographic features of a 37-year-old male with a painless palpable mass in the right groin that was identified as dedifferentiated retroperitoneal liposarcoma herniated through the right inguinal canal.
Liposarcomas are the most common primary soft-tissue malignancies of the retroperitoneum. They are divided into five subtypes: well-differentiated, dedifferentiated, myxoid, pleomorphic, and mixed, according to the 2002 World Health Organization histological classification (1). Well-differentiated retroperitoneal liposarcoma is the most common subtype and is considered a low-grade tumor. The dedifferentiated subtype is a transition from well-differentiated liposarcoma to nonlipogenic sarcoma with variable histological grades. Although several journals have reported retroperitoneal liposarcomas presenting as indirect inguinal hernias, dedifferentiated liposarcoma extending into inguinal canal and seemingly presenting as inguinal hernia has been more scarcely described (23). Here we report multidetector computed tomography (CT) and magnetic resonance imaging (MRI) findings of a dedifferentiated retroperitoneal liposarcoma mimicking a right inguinal hernia.
A 37-year-old male visited our hospital with a painless protruding mass in the right inguinal region. The patient had no other symptoms or relevant past medical history. Physical examination revealed a palpable mass without tenderness in his right groin. His laboratory findings were unremarkable.
Contrast-enhanced abdominal CT examination using a 128-detector-row CT scanner (Definition AS+, Siemens Medical Solutions, Forchheim, Germany) revealed a predominant fat-density mass with enhancing septa and a few solid nodules measuring approximately 12 × 7.5 cm (Fig. 1). The caudal portion of the mass, located in the right inguinal canal, mainly consisted of soft tissue with an enhancement measuring about 3.5 × 11 cm in size. The tumor anteriorally displaced the ascending colon and mesocolon and extended alongside the right testicular vessels, suggesting an extraperitoneal location of the lesion. Also, the mass passed between the right inferior epigastric vessels medially, and the right external iliac vessels laterally.
Pelvic MRI was performed using a 3.0T system (Magnetom Verio; Siemens Medical Solutions, Erlangen, Germany) with a body phased-array coil, in order to obtain more information. On MR images (Fig. 1), most of the retroperitoneal mass showed high signal intensity on T1-weighted images and intermediate to high signal intensity on T2-weighted images, and demonstrated a signal drop on fat-saturated images. The irregular septa and nodules, which appeared inonly a small part of the retroperitoneal area, were of low signal intensity on T1-weighted images and were enhanced slightly after administration of gadolinium pentetic acid (Gd-DTPA, Magnevist®; Bayer Healthcare Pharmaceuticals, Wayne, NJ, USA). In the right inguinal canal, however, the majority of the mass showed low signal intensity on T1-weighted images and high signal intensity on T2-weighted images with profuse contrast enhancement following Gd-DTPA injection.
The patient underwent surgical resection of the mass including components of both theretroperitoneal and the right inguinal canal. Intraoperatively, predominantly fatty masses containing hard, solid lesions with multiple septa were found in the retroperitoneal space. The firm mass in the right inguinal area had clearly originated from the large retroperitoneal mass and broken through the right inguinal canal.
Histopathologically, the surgical specimen was diagnosed as a well-differentiated retroperitoneal liposarcoma with a dedifferentiated component. Grossly, the tumor was unencapsulated, well-circumscribed, oval, rubbery, and about 8.0 × 5.0 × 4.0 cm in size. The cut surface of the intracanalicular portion was solid with discrete intratumoral nodules varying in size. Its color ranged from yellow to yellow-tan admixed with tan-gray areas that corresponded to the dedifferentiated foci. There was no evidence of hemorrhage or necrosis (Fig. 1G). Viewed microscopically, the tumor had two different histological components separated by either an abrupt or gradual transition. The well-differentiated area was characterized by proliferating adipocytes varying in size and shape, whereasthe dedifferentiated area was characterized by spindle cells arranged in whorls and fascicles (Fig. 1H). Most of the dedifferentiated area had the appearance of a high-grade malignant fibrous histiocytoma or a rhabdomyosarcoma with different degrees of chronic inflammation and fibrosis. The tumor cells showed marked nuclear atypia and pleomorphism with abundant, finely granular, amphophilic to basophilic cytoplasm in bipolar, stellate, or polygonal configurations, and vesicular nuclei with one to a few prominent nucleoli. The mitotic count was 1–2 mitotic fields/10 high-power fields in the most mitotically active area. Immunohistochemistry demonstrated strong expression of vimentin and α-1-antitrypsin and negative staining for muscle markers and S100 protein.
The inguinal canal is involved in a broad spectrum of pathological processes including inguinal hernia, other congenital disease, inflammatory conditions, and even neoplasm, manifesting as palpable groin mass. The incidence of malignancy is about 30% in neoplastic disease involving the inguinal canal. The majority of malignant inguinal tumors consist of mesenchymal sarcomas such as liposarcoma (4). Dedifferentiated liposarcoma, one of five liposarcoma subtypes, originates from well-differentiated liposarcoma with an abrupt transition to aggressive, high-grade nonlipogenic sarcoma. According to Lahat et al. (5), dedifferentiated liposarcomas are likely to recur as local or distant metastatic disease and their treatment accordingly requires intensive multimodal approaches. The presence of a nonfatty component within a predominantly fatty mass or a well-defined fatty mass within a well-defined nonfatty mass are often suggestive of dedifferentiated liposarcoma (16). Relative to muscle, these masses are hypointense on T1-weighted MRI, but are heterogeneously hyperintense on T2-weighted MRI (7). Taking all of this into account, we preoperatively diagnosed the tumor as a retroperitoneal liposarcoma containing a dedifferentiated component.
The diagnosis of retroperitoneal tumors is often challenging for radiologists. The most important clue is whether the tumor is located within the retroperitoneal space. Displacement of adjacent retroperitoneal organs or major vessels in the retroperitoneal cavity, observed using either CT or MRI, is a useful sign. Tracing the tumor's spread and characterizing its contents can also narrow the possible diagnoses (8). In our patient, we concluded that the tumor was located in the retroperitoneal space because the ascending colon and its mesocolon were displaced anteriorly, and the mass had spread alongside the testicular vessels. The retroperitoneum is known to have contact with the pelvic prevesicular space (9). The anterolateral segments of the testicular vessels travel within this space before entering the internal inguinal ring to become part of the spermatic cord. Prevesicular fat accompanying the testicular vessels is surrounded by the internal spermatic fascia, which arises from the transversalis fascia.
Incarcerated omental inguinal hernias could mimic well-differentiated or dedifferentiated liposarcomas extending into the inguinal canal not only in clinical manifestation, but also in imaging features (10). Both of them usually present as irreducible, palpable groin masses with varying degrees of nonfatty or scanty fatty components on imaging studies. However, unlike herniated retroperitoneal liposarcomas, incarcerated omental inguinal hernias originate in omental arteries and have no relation to testicular vessels.
Knowledge of both the imaging findings of dedifferentiated liposarcoma and the retroperitoneal anatomy is critical to correct preoperative diagnosis and treatment planning in cases of retroperitoneal liposarcoma.
Figures and Tables
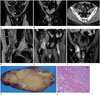 | Fig. 1A 37-year-old male with a retroperitoneal dedifferentiated liposarcoma (DDL) extending into the right inguinal canal.
A, B. Contrast-enhanced computed tomography (CT) with coronal reformatted images shows a large mass with a fatty component in the retro-peritoneum (thin arrows) extending into the right inguinal canal with a solid enhancing component (open arrow). Note the medial displacement of the inferior epigastric vessels (arrowhead).
C. Axial contrast-enhanced CT demonstrates several ill-defined strands and a soft-tissue nodule with homogenous enhancement (open arrow) within the mass. Note the mass adjacent to and extending along the testicular vessels (arrowhead).
D. Sagittal T2-weighted image of the retroperitoneal tumor extending into the right inguinal canal. The retroperitoneal component of the mass shows high signal intensity consistent with mature fatty tissue (white arrows), while the inguinal canal component shows intermediate signal intensity consistent with tumor tissue (black arrow).
E, F. Coronal gadolinium-pentetic-acid-enhanced T1-weighted images demonstrate multiple tenuous septa (arrows) and a soft-tissue nodule with marked enhancement (open arrow) within the mass.
G. On cross-section, the surgical specimen taken from the right inguinal canal is solid, with discrete intratumoral nodules of varying size and colors ranging from yellow to yellow-tan admixed with firm tan-gray areas corresponding to dedifferentiated foci (open arrows).
H. The tumor shows an abrupt transition between the components of well-differentiated liposarcoma and DDL (hematoxylin & eosin, × 100).
|
References
1. Hong SH, Kim KA, Woo OH, Park CM, Kim CH, Kim MJ, et al. Dedifferentiated liposarcoma of retroperitoneum: spectrum of imaging findings in 15 patients. Clin Imaging. 2010; 34:203–210.
2. Mizuno Y, Sumi Y, Nachi S, Ito Y, Marui T, Saji S, et al. A case of a large retroperitoneal liposarcoma presenting as an incarcerated inguinal hernia. Hernia. 2006; 10:439–442.
3. McKinley SK, Abreu N, Patalas E, Chang A. Large Retroperitoneal Liposarcoma Diagnosed upon Radiological Evaluation of Mild Right-Sided Inguinal Hernia. Case Rep Radiol. 2013; 2013:187957.
4. Vagnoni V, Brunocilla E, Schiavina R, Borghesi M, Passaretti G, Gentile G, et al. Inguinal canal tumors of adulthood. Anticancer Res. 2013; 33:2361–2368.
5. Lahat G, Anaya DA, Wang X, Tuvin D, Lev D, Pollock RE. Resectable well-differentiated versus dedifferentiated liposarcomas: two different diseases possibly requiring different treatment approaches. Ann Surg Oncol. 2008; 15:1585–1593.
6. Tateishi U, Hasegawa T, Beppu Y, Satake M, Moriyama N. Primary dedifferentiated liposarcoma of the retroperitoneum. Prognostic significance of computed tomography and magnetic resonance imaging features. J Comput Assist Tomogr. 2003; 27:799–780.
7. Song T, Shen J, Liang BL, Mai WW, Li Y, Guo HC. Retroperitoneal liposarcoma: MR characteristics and pathological correlative analysis. Abdom Imaging. 2007; 32:668–674.
8. Nishino M, Hayakawa K, Minami M, Yamamoto A, Ueda H, Takasu K. Primary retroperitoneal neoplasms: CT and MR imaging findings with anatomic and pathologic diagnostic clues. Radiographics. 2003; 23:45–57.
9. Aikawa H, Tanoue S, Okino Y, Tomonari K, Miyake H. Pelvic extension of retroperitoneal fluid: analysis in vivo. AJR Am J Roentgenol. 1998; 171:671–677.
10. Aguirre DA, Santosa AC, Casola G, Sirlin CB. Abdominal wall hernias: imaging features, complications, and diagnostic pitfalls at multi-detector row CT. Radiographics. 2005; 25:1501–1520.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download