Abstract
Clear cell ependymoma (CCE) is a histological rare variant (1–5%) of ependymoma, which is distinguished from other histological subtypes by the presence of fusiform cells arrayed radially around small blood vessels. These alleged perivascular pseudorosettes are significant characteristic features of ependymomas. About 95% of infratentorial ependymomas are found in the fourth ventricle and the remainder occurs as cerebellopontine angle lesions. In previous reports, the cerebellum is found to be a rare location for ependymoma. In this study we report one case of CCE originating from the cerebellar hemisphere, showing unusual morphology on 3T MRI.
Ependymomas are glial tumors that share histological features of ependymal differentiation. In 2007, ependymomas have been classified by the World Health Organization neuropathology groups into Grades I, II, and III. Grade I tumors include myxopapillary ependymomas and subependymomas. Grade II tumors are designated by the name ependymoma, whereas Grade III tumors are called anaplastic ependymoma. There are four variants: cellular, papillary, clear cell and tanycytic ependymomas; they usually are classified as Grade II or III.
Clear cell ependymoma (CCE) is recognized lately as a distinctive form of ependymomas and it reveals typical pathologic diagnostic features. This variant of ependymoma was found to have a predilection for supratentorial compartment and it may be misdiagnosed as oligodendrogliomas or central neurocytoma and even metastatic renal cell carcinoma (1). CCE can be presented as cystic mass with mural nodule (12). However, this report is about CCE in the cerebellar hemisphere showing predominantly cystic features on 3T MRI.
A 10-year-old girl presented with an intermittent headache for 3 weeks duration. She also had mild degree of nausea and dizziness in the morning. There is no family history of migraine and no history for abdominal pain or fever. Her body weight and height were 39 kg and 140 cm respectively. Her blood pressure was 120/70 mm Hg. During the first visit to pediatric department, there were no abnormal physical signs or neurologic signs. Contrast-enhanced CT showed 5.2 × 4.5 cm sized low-density mass with marginal enhancement at left cerebellar hemisphere (not shown). The 4th ventricle was compressed and displaced to right anterior aspect because of the mass resulting in obstructive hydrocephalus. The first radiological diagnosis was pilocytic astrocytoma. On her second visit to the Pediatric Department, she was still suffering from intermittent headache. There was dysmetria on the performance of a finger-to-nose test. Because of the very large cyst size and obliteration of the cisterna magna, she underwent emergency operation with a burr hole craniotomy and cyst drainage. MR images revealed that there was a cystic mass in the left cerebellar hemisphere with a high signal intensity wall on T2-weighted imaging (T2WI) and fluid attenuation inversion recovery (FLAIR) pulse sequences. The mass showed subtle rim enhancement after contrast on T1-weighted imaging (T1WI) and there was no definite mural nodule (Fig. 1). It was also associated with obstructive hydrocephalus.
The mass was excised under navigation guidance, via the left sub-occipital approach. The mass had yellow-whitish color, vague boundary line, and showed adhesion to the surrounding brain tissue. At cystic portion of the mass, the protein rich material of yellowish clear-color was observed. The tumor was partially removed because of the vague boundary line and serious adhesion. After 3 months later, the patient was hospitalized for resection of the remaining left cerebellar mass. She had neither dizziness nor headaches and her cerebellar function was intact. During the second operation, the tumor was near totally removed and the small portion around the vermis was intentionally left, so as not to result in neurological deficits such as truncal ataxia and cerebellar dysfunction.
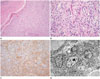
On routine hematoxylin and eosin staining, the case revealed a distinct margin in connection with the adjacent brain and perivascular pseudorosettes, which is a characteristic feature of conventional ependymoma. On immunohistochemistry, it was positively labeled for glial fibrillary acidic protein (GFAP) but nevertheless the epithelial membrane antigen (EMA) was negative. On ultrastructural examination, some microvilli within intracytoplasmic lumina were recognized (Fig. 2).
The patient received radiation treatment after surgery (60 Gy/30 fxs) and she is still doing well without any neurologic symptoms.
Ependymoma accounts for less than 10% of primary central nervous system (CNS) tumors. The intramedullary ependymomas usually occur in adults, whereas the majority of intracranial ependymomas are found in children (2). CCE is a very rare brain tumor, constituting around 0.3% of all gliomas (3). Sixty-two cases of CCE have been reported from 1989 to 2009. CCE is an unusual tumor of the CNS with a predilection for the supratentorial compartment in children (4). The average age at diagnosis was 7.5 years and the incidence of ependymoma is slightly higher in males than in females. These epidemiological characteristics are similar to our case. During the period of 2004–2006, in children aged 0–14 years, ependymomas constituted 6.5% of all tumors diagnosed from approximately 500 children diagnosed with brain tumors (5).
In general, 60–70% of ependymomas occur in the infratentorial compartment, and of which 95% are seen in the fourth ventricle (2). But, the supratentorial CCE is an uncommon variant ependymoma that is dissimilar to conventional ependymomas (3). Infratentorial CCEs are commonly found in the cerebellum (67%) (4).
Tumor cells may spread along the spinal cord but rarely to intracranial places; spreading to the spine is more likely in infratentorial tumors than in supratentorial ones. Ependymomas rarely spread outside of the CNS. In our case, there was no clinical evidence related to extracranial metastases. Symptoms of ependymoma may be different in relation to the location and size of the tumor. Vomiting and headache are the most frequent symptoms in older children and adults. These are the usual signs associated with hydrocephalus. Headaches are generally worse in the morning. The patient in this report showed similar clinical symptoms.
CCEs, which show clear cytoplasm with a perinuclear halo, can be seen in some cases but they were primarily interpreted as oligodendrogliomas. However, in 1989, Kawano et al. (1) described CCE as a distinct pathological entity and these clear cells are ependymal cells of neoplastic variation. This is proved by an electron microscopic examination and it exhibits typical features, such as well-developed intercellular junctions, microvilli, and cilia (2). On routine histological inspection (Fig. 2), CCE is characterized by sheet of uniform tumor cells with a perinuclear halo, and centrally located rounded nuclei. This feature may mimic oligodendroglioma. Unlike oligodendrogliomas, which are diffusely infiltrative, ependymomas, including the clear cell variety, generally are sharply circumscribed. The present case also showed a discrete margin in relation to adjacent brain. Perivascular pseudorosettes are a key architectural feature of conventional ependymoma, however, CCE may be subtle, like the present case. Perivascular rosettes may be inconspicuous, and GFAP may be needed to highlight them (6). CCEs frequently show an expression of GFAP in the fibrillary matrix and a cytoplasmic punctuate, ring-like positivity for EMA. However, ependymomas can be distinguished from these tumors at the histo-immumochemical level as ependymomas are always positively labeled for GFAP, at least in the perivascular rosettes areas. The absence of GFAP staining should prompt a search for another diagnosis (6). The present case showed GFAP positivity, however EMA was negative. On ultrastructural examination, all ependymal tumors, including CCE, share similar characteristics. These include intercellular junction complex and microvilli, the latter present both on cell surfaces and within microlumina; however, oligodendrogliomas lack microvilli, and intercellular junctional complexes. In the present case, some microvilli within intracytoplasmic lumina were identified. Taken together the present tumor was diagnosed with CCE despite of its ambiguous morphology.
On conventional MR images, intracranial ependymomas generally show hypointense or isointense on T1WI, and enhance heterogeneously following gadolinium DTPA administration (7). They are usually hyperintense on T2WI and FLAIR pulse sequences because of high fractions of tumor with regions of cyst formation or intracellular myxoid formation (7). On T2 FLAIR imaging, cystic areas of a tumor are seen as same density with fluid but may remain hyperintense because of proteinaceous materials in the fluid (8). In approximately 50% of the cases, calcifications can be seen on CT (78). Diffusion-weighted imaging usually reveals reduced diffusion within the soft tissue components of some ependymomas. This has been attributed to the high cellularity of some lesions (8).
CCE presents with various sizes in different locations with hypercellularity, that is, isointense/isointense or isointense/hypointense to gray matter on T1WI and T2WI. Hemorrhage, necrosis, vasogenic edema, mass effect, and calcifications are related to some tumors (4). In our case, based on MRI features, we considered pilocystic astrocytoma as a first diagnosis. Pilocystic astrocytomas are found in the posterior fossa, showing strongly contrast enhanced nodules, and proteinaceous fluid containing cystic component, which follows the cerebrospinal fluid signal on conventional MRI (9). They are well demarcated from the surrounding brain tissue (9), but our case showed a vague border between the tumor mass and the surrounding brain parenchyma. In some cases, apparent diffusion coefficient values can be helpful in differentiating these two tumor types (9). The highly vascular ependymoma can mimic hemangioblastoma (1).
CCE lacks a characteristic ependymomatous area, so it is difficult to make sure of the diagnosis only by histologic examination. Immunostaining and electron microscopic verification are needed to confirm the diagnosis (1). Hemangioblastoma itself is a highly vascular tumor and may present as a large cystic cavity with a mural nodule (45%) or a purely solid tumor (45%) (10). On T2WI, tubular flow voids can be found since they have large feeding and draining vessels in the periphery and within the solid component (10). This feature is not present in our case and hemangioblastomas are extremely rare in children younger than 18 years old with an incidence of less than 1 per one million, so the possibility of hemangioblastoma is low. However, the surgical outcome of hemangioblastoma is favorable as it is a biologically benign tumor. 201TI-SPECT can be used to differentiate CCE.
Figures and Tables
Fig. 1
Precontrast CT (A) and postcontrast CT (B) show cystic mass in the left cerebellar hemisphere with thin enhancing wall without gross hemorrhage or calcification. Axial T2-weighted (C) and T2 fluid attenuation inversion recovery (D) 3T MR images show cystic mass in the left cerebellar hemisphere with thin high intensity wall. Axial and sagittal contrast-enhanced T1-weighted images (E, F) show thin marginal enhancement without definite mural nodule. Diffusion weighted image (G) shows no restricted diffusion with high apparent diffusion coefficient value (H).
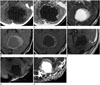
Fig. 2
Photomicrographs of the excised cerebellar mass.
A. Photomicrographs show tumor cells arranged in sheets intersected by small blood vessels. Discrete margin is noted in relation to adjacent brain (H&E, × 100).
B. Tumor cells show rounded nuclei and abundant surrounding clear cytoplasm. Perivascular pseudorosettes are subtle (H&E, × 400).
C. Tumor shows positivity for glial fibrillary acidic protein in the fibrillary matrix (× 200).
D. Electron photomicrograph shows intracytoplasmic lumina with some microvilli (× 4000).
H&E = hematoxylin and eosin
References
1. Kawano N, Yagishita S, Komatsu K, Suwa T, Oka H, Utsuki S, et al. Cerebellar clear cell ependymoma mimicking hemangioblastoma: its clinical and pathological features. Surg Neurol. 1999; 51:281–287. discussion 287-288
2. Kim JH, Cho BK, Kim IO, Park SH. Clear-cell ependymoma of the cerebellum: a case report. Ultrastruct Pathol. 2007; 31:241–247.
3. Min KW, Scheithauer BW. Clear cell ependymoma: a mimic of oligodendroglioma: clinicopathologic and ultrastructural considerations. Am J Surg Pathol. 1997; 21:820–826.
4. Fouladi M, Helton K, Dalton J, Gilger E, Gajjar A, Merchant T, et al. Clear cell ependymoma: a clinicopathologic and radiographic analysis of 10 patients. Cancer. 2003; 98:2232–2244.
5. Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013; 15:Suppl 2. ii1–ii56.
6. Godfraind C. Classification and controversies in pathology of ependymomas. Childs Nerv Syst. 2009; 25:1185–1193.
7. Lefton DR, Pinto RS, Martin SW. MRI features of intracranial and spinal ependymomas. Pediatr Neurosurg. 1998; 28:97–105.
8. Yuh EL, Barkovich AJ, Gupta N. Imaging of ependymomas: MRI and CT. Childs Nerv Syst. 2009; 25:1203–1213.
9. Pavlisa G, Pavlisa G, Rados M. Diffusion differences between pilocytic astrocytomas and grade II ependymomas. Radiol Oncol. 2011; 45:97–101.
10. Plaza MJ, Borja MJ, Altman N, Saigal G. Conventional and advanced MRI features of pediatric intracranial tumors: posterior fossa and suprasellar tumors. AJR Am J Roentgenol. 2013; 200:1115–1124.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download