Abstract
In this review, we present computed tomography (CT) and magnetic resonance imaging (MRI) findings of various calvarial lesions on the basis of their imaging patterns and list the differential diagnoses of the lesions. We retrospectively reviewed 256 cases of calvarial lesion (122 malignant neoplasms, 115 benign neoplasms, and 19 non-neoplastic lesions) seen in our institutions, and classified them into six categories based on the following imaging features: generalized skull thickening, focal skull thickening, generalized skull thinning, focal skull thinning, single lytic lesion, and multiple lytic lesions. Although bony lesions of the calvarium are easily identified on CT, bone marrow lesions are better visualized on MRI including diffusion-weighted imaging or fat-suppressed T2-weighted imaging. Careful interpretation of calvarial lesions based on pattern recognition can effectively narrow a range of possible diagnoses.
In this article, we reviewed our institutional database and radiology department database of images taken from 2005 to 2013 and retrieved the records of 256 patients who underwent brain CT or MRI and were later diagnosed with tumorous or nontumorous calvarial lesions (122 malignant neoplasms, 115 benign neoplasms, 19 non-neoplastic lesions). The majority of patients either had multiple myelomas (n = 108, 42.2%) or benign osteomas (n = 99, 38.7%) (Table 1). Though calvarial metastases are the most common malignant lesions, multiple myeloma is more common in our database because of errors in coding and also because our institution is renowned for treating hematologic malignancies. We retrospectively reviewed the imaging findings of CT and MRI for these calvarial lesions, and, using a categorical imaging approach, we briefly described and illustrated the imaging findings of representative osteolytic calvarial lesions. The Institutional Review Board approved this retrospective review, and the requirement of informed consent was waived.
With increasing frequency, CT and MRI have been incorporated into the basic imaging tools for evaluating calvarial lesions. Therefore, it is useful to categorize diagnostic features of images taken not by conventional radiography but by CT and MRI (12). It is possible to categorize 8 different types of calvarial lesions based on 4 key features: thickening or thinning of calvarial bone, sclerosis or lysis of bone, focal or generalized lesions, and, finally, singularity or multiplicity of lesions. On CT and MRI, sclerotic lesions appear as thickening of calvarial bone (either the inner/outer tables, or bone marrow, or both). Accordingly, we can simplify the classification system into six categories based on three main features. We suggest that calvarial lesions are classified into calvarial thickening, thinning, and lytic lesion. When the calvarial lesions fall into the 'thickening' or 'thinning' categories, they can be further subdivided into focal or generalized lesions. The lytic category can be subdivided into either single or multiple lesions. Furthermore, we can expect to get additional information about matrix characteristics with contrast enhancement and diverse advanced MR sequences such as diffusion-weighted imaging (DWI) (3), perfusion-weighted imaging, and MR spectroscopy. A flowchart for a systematic approach to imaging diagnosis of calvarial lesions is provided in Table 2.
Multiple myeloma is characterized as multiple osteolytic lesions due to the malignant proliferation of osteoclast-activating factors triggered by myeloma. Plain radiography of multiple myeloma patients is characterized by a subcortical circular or elliptical radiolucent shadow, and the axial skeleton is the predominant site of the abnormality. CT shows punched-out lesions with soft tissue masses and fractures. On MRI, multiple myeloma presents with low-to-intermediate signal intensity on T1-weighted images, high signal intensity on T2-weighted images, and high signal intensity on DWI (4). The lesions enhance strongly following contrast administration. Four different imaging patterns may appear: normal-looking marrow, a micro nodular pattern (also described as variegated or salt and pepper lesions), a focal pattern, or a diffuse pattern (Fig. 1) (12).
Metastases take various forms; they can be seen as either osteoblastic or osteolytic lesions on plain radiograph. When appearing as single or multiple lytic lesions, metasteses are usually seen as one or more relatively circumscribed intraosseous lesions or sometimes as diffusely destructive on bone algorithm CT (2). Hypointense infiltrating foci are seen on T1-weighted images as metastases replace hyperintense normal yellow marrow. Most skull metastases are hyperintense compared to bone marrow on T2-weighted images and appear clearly on fat-saturated contrast-enhanced T1-weighted images (Fig. 2). DWI is helpful for the detection of bone marrow involvement of the calvarium (3).
Langerhans cell histiocytosis (LCH) is an uncontrolled monoclonal proliferation of abnormal Langerhans cells involving any organ. When LCH involves the skull, it manifests as a small soft tissue calvarial mass with lytic defect and beveled edge more on the inner table than the outer table on plain radiograph and CT scans. It is often seen as bilateral soft tissue masses with bone destruction. On MRI, LCH presents as an enhancing soft tissue mass on contrast-enhanced T1-weighted images with vivid enhancement of the pituitary infundibulum and stalk thickening (Fig. 3) (567).
Osseous hemangiomas are benign skull lesions composed of predominantly vascular entities. On plain radiographs and CT scans, they present as sharply defined expansile lesions, which may include a periosteal reaction and be surrounded by a thin peripheral sclerotic rim, as is the case approximately 30% of the time. They show intact inner and outer tables; the outer table tends to be more expanded than the inner table. Trabecular thickening with radiating spicules is also commonly seen. MRI signal strength depends on the quantity of slow-moving venous blood, the proportion of red to fatty marrow, and hypointense trabeculae (8). Osseous hemangiomas are typically hyperintense on T1-weighted images and heterogeneously hyperintense on T2-weighted images. They enhance intensely, heterogeneously, or minimally on contrast-enhanced CT or MRI (Fig. 4) (89).
Epidermoid and dermoid cysts are ectodermal inclusion cysts lined by epithelium. They occur inside the orbit, in the calvarial diploic space, and intracranially (9). They typically appear as encapsulated unilocular cystic lesions with a signal intensity that depends on their components (1). On plain radiograph and CT scans, epidermoids appear as intradiploic, expansile, osteolytic lesions with smooth sclerotic margins while dermoids appear as expansile, osteolytic midline lesions. On CT, dermoids contain a soft-tissue component extending to overlying skin and intracranial areas. Usually epidermoids have a fluid-like signal intensity on MRI. Dermoids have a heterogeneous appearance with varied signal intensities depending on their various compositions. Since dermoids contain fatty tissue, they show low attenuation on CT and high signal intensity on T1-weighted images (Fig. 5) (169). Enhancement is generally absent although mild peripheral enhancement can be seen in about 25% of cases.
When a meningioma invades overlying bone, it can create an osteolytic bone lesion. Infrequently, meningioma arises initially in the calvarium. It is thought that trapping of ectopic meningocyte or arachnoid cap cells in the cranial sutures may cause primary intraosseous meningiomas (10). Approximately 68% of primary extradural meningiomas involve the calvarium (11). Primary extradural meningiomas typically present with slow scalp swelling and do not show any neurologic symptoms or signs, unless the lesion extends through the inner table and compresses intracranial structures (12). On plain radiograph and CT, well-defined radiolucent, osteolytic lesions can be seen. On MRI, meningiomas are usually hypo- to isointense on T1-weighted images and iso- to hyperintense or sometimes heterogeneously intense on T2-weighted images. Contrast enhancement generally enhances evenly and often produces a dural tail (Fig. 6) (2).
Fibrous dysplasia is characterized as the replacement of normal cancellous bone with abnormal fibrous tissue (13). On plain radiograph, they appear as ground-glass sclerotic lesions or cystic lesions commonly crossing bony sutures. They may involve multiple calvarial bones (14), tending to cause the outer table to bulge while conserving the shape of the inner table (15). CT scans show a degree of hazy and intradiploic density. On T1-weighted images, they are usually hypointense, but more fibrous areas can be isointense. On T2-weighted images, they show various signals, depending on their cellularity: those that are more fibrous with fewer bony trabeculae and less cellularity exhibit relatively high signal intensities, while more osseous and cellular matrices can show relatively low signal intensities (16). Contrast enhancement produces a variable range of intensities depending on lesion stage; there can be homogeneous, central or peripheral enhancement (Fig. 7).
Malignant lymphoma may originate in the skull and extend to outside the cranium (17). Presenting as nonspecific osteolytic lesions on plain radiograph, on CT scans they instead appear as extra-intra cranial isodense lesions in the cranial vault or homogenous masses with sharp margins. On MR, most reported cases of skull lymphomas produced isointense signals on pre-contrast T1-weighted images with intense enhancement with contrast. However, MR imaging does not diagnose lymphoma accurately because it can mimic metastatic carcinoma, osteomyelitis, or in some cases, meningioma (Fig. 8) (18).
Desmoplastic fibroma is a rare benign bone tumor which originates from fibrous tissue, and a majority of cases are seen in those younger than 30 years of age. Desmoplastic fibroma most commonly occurs in the metaphyses of the long bones, the mandible, and the pelvis, whereas the maxilla, calvaria, sternum, and vertebrae are less frequently affected. Simple radiographs show an expansile, osteolytic lesion with a trabeculated or bubbly appearance. At times, the tumor may have a very aggressive radiographic appearance with bone destruction, cortical erosion, or soft-tissue invasion (1920). The dense connective tissue and hypercellular regions have intermediate signal intensity on T1-weighted images and heterogenous signal intensity on T2-weighted images (Fig. 9) (19).
Atretic cephalocele is composed of dura, fibrous tissue, and dysplastic brain tissue. Oval or elongated defects are observable on plain skull radiographs. On CT scans, a subgaleal soft tissue mass with "spinning top" configuration is seen. On MRI, a subcutaneous scalp mass with intracranial extension through skull and dura may be seen as having heterogeneous signals on T1-weighted images and high signals on T2-weighted images and heterogeneous enhancement after contrast injection. On MR venography, a vertically positioned straight sinus equivalent (persistent falcine vein) is seen (Fig. 10) (21).
Gorham disease is an intraosseous neoplastic proliferating disease of hemangiomatous or lymphagiomatous tissue with progressive massive osteolysis. The first stage involves vascular proliferation in connective tissue; in the second stage, fibrous tissue replaces the absorbed bone without regeneration of the bone matrix. On CT scans, radiolucent foci in the intramedullary or subcortical regions are seen and the gradual disappearance of bone may be apparent. MR imaging of Gorham disease involving the calvaria has not been widely reported, but a reticular pattern on a contrast enhanced fat-suppressed T1-weighted image was reported (16).
Burr holes appear as well-defined defects in the inner and outer tables of the skull vault on CT or simple radiograph. On contrast-enhanced MR images, the margins of the burr hole usually show enhancement, and in some cases, diffuse enhancement with a filling of the plunge defect may be seen. Defects caused by plunging may show high signal intensity on T2-weighted images, which is referred to as a "mushroom sign" (22).
Skull osteosarcomas show nonspecific radiological features, for example, they can be either osteolytic, osteoblastic, or mixed in involvement pattern. However, spicular calcification tends to be seen, and a sun burst appearance exists in 25% to 31% of cases (23). Bone destruction and mineralization of the tumors may indicate an osteogenic sarcoma (24).
Osteomyelitis of the calvarium may occur as a consequence of trauma or as a complication of sinus sepsis. Pus in the bone can spread either into the cranium or outward into subgaleal or subcutaneous planes where it can form abscesses, visible as osteolytic lesions on CT (25).
Arachnoid granulations usually present as sharply marginated, lucent, occipital bone lesions adjacent to the transverse sinus. They generally appear as hypointense or isointense relative to the brain parenchyma on T1-weighted images and hyperintense on T2-weighted images and show minimal heterogeneous contrast enhancement (2627).
Neurofibromatosis type 1 is a multisystem neurocutaneous disorder that is one of the most common inherited autosomal dominant central nervous system disorders. The radiographic spectrum includes focal areas of abnormal signal intensity in deep grey or white matter, optic nerve glioma, progressive sphenoid wing dysplasia, lambdoid suture defects, dural calcification at the vertex, buphthalmos, and in rare cases, the Moyamoya phenomenon (28).
Osteoblastic metastases arise from hematogenous spread from primary cancers. In such instances, they are often seen without benign sclerotic borders. On CT scans, metastatic lesions are usually seen as enhancing masses centered in bone with osseous destruction and most are lytic, though a few are sclerotic (e.g., prostate lesions). Osteoblastic metastases appear as hypointense lesions on both T1-weighted and T2-weighted images without significant enhancement and appear positive on DWI (229).
Osteomas are benign, slow-growing tumors usually found incidentally (30). They are the most common benign osseous tumors in the calvarium, and are mainly found in the outer table of the skull (31). They appear as dense oval lesions on radiographs. On CT images, they appear as very hyperdense sclerotic masses with clear-cut outlines that grow from, and attach to, the external aspect of the cortical bone. They are homogeneously hypointense on T1-weighted images, heterogeneously hypointense on T2-weighted images, and do not show contrast enhancement (2).
Fibrous dysplasia can be seen as calvarial thickening because it tends to cause the outer table to bulge while conserving the shape of the inner table (15).
Usually benign, meningiomas arising from the dura can cause hyperostosis. On plain radiograph and CT, 75% of cases appear as round or smoothly lobulated masses that are hyperdense compared to the cerebral cortex. 25% of cases demonstrate calcification. Hyperostosis on bone algorithm CT is often seen (32).
Growth hormone hypersecretion can manifest as generalized thickening of the skull. Bony abnormalities associated with growth hormone include sella turcica alterations, prominence and enlargement of frontal and maxillary sinuses, excessive pneumatization of the mastoids, prominence of the occipital protuberance, thickening or, less commonly, thinning of the cranial vault, enlargement and elongation of the mandible, and widening of the mandibular angle (32).
Chronic intracranial hypotension or prolonged ventricular shunting for hydrocephalus can cause diffuse thickening of all bones of the neurocranial vault. This happens because outward pressure needed to expand the cranium is missing, so the inner table of the skull bone grows instead. On simple radiographs and CT scans, the entire cranial vault thickening can be seen (33).
Dilatin (Diphenylhydantoin) is widely used for the management of patients with epileptic convulsions. Dilatin has recently been shown to stimulate osteoblast proliferation in approximately 34% of patients. The degree of skull thickening is correlated with frontal bossing and degree of facial coarsening (34). The increase in the thickness of the calvarium is mainly presented in the diploic space (35).
Paget disease, also known as osteitis deformans, can cause chronic metabolic skeletal disorders (2) characterized by bony expansion with variable destruction with or without sclerosis. The characteristics of CT and MR are a loss of normal trabeculae from lysis, a disorganized pattern of trabecular thickening, and, finally, sclerosis when the disease is in the blastic phase. Since a majority of cases are seen in the mixed phase, the yellow marrow signal intensity is maintained regardless of the MR sequence (3637).
Patients with hemolytic anemia have red marrow hyperplasia, causing the widening of the diploic space; the outer table thins or is completely obliterated. Before diploic widening, a coarse granular or stippled pattern can be seen in the upper parietal region. When the hyperplastic marrow perforates or destroys the outer table, new bony spicules perpendicular to the inner table are seen as "hair-on-end" signs (38).
Cephalhematoma that is not absorbed within a month begins to ossify where the periosteum is lifted and the ossification gradually covers the surface of the hematoma. The thick band-like ossification mimics doubling of the skull, so it is referred to as a "double skull sign" (39). On CT and radiograph, it appears as a uniform, densely calcified mass located immediately adjacent to the outer table, within the periosteum (1).
Hyperostosis frontalis interna is a benign condition which thickens the inner table of the frontal bone. Though the etiology is not clearly known, estrogen signaling is thought to be involved. The hyperostotic bone tends to be less dense on simple radiographs or on CT than are normal cortical bones (4041).
Prominent convolutional marking is widely considered to be a reflection of normal brain growth. The growing brain exerts a continuous pulsatile pressure on the cranium, producing a gyral pattern on plain radiographs which is known as the copper-beaten pattern (42).
Craniosynostosis is a rare, complex condition defined as a premature fusion of one or more of the cranial sutures (43). Primary craniosynostosis is due to a developmental error during embryogenesis, while secondary craniosynostosis is due to mechanical or metabolic causes (44). In plain radiographs, perisutural sclerosis, localized breaking, bony bridging, and absence of the suture are primary signs of craniosynostosis. When raised intracranial pressure is maintained over long, fingerprinting and copper beating can be seen (45).
Lacunar skull is characterized as a dysplasia of the membranous bone caused by mesenchymal abnormality and is associated with neural tube defects, especially myelomeningocele with Chiari II malformation. It is seen as a well-defined lucent lesion (having a scooped-out appearance) which corresponds to the nonossified fibrous bone (32).
Regardless of the cause of hydrocephalus, increased intracranial pressure may result in diffuse calvarial thinning with or without deformity when it is not treated properly (33).
Parietal thinning is an uncommon condition which can be caused by either non-progressive congenital dysplasia of the dipole or acquired and progressive diseases such as tumors, diabetes, long-standing steroid therapy, or osteoporosis. The bilateral parietal bones show symmetrical thinning (involving the outer tables and diploe) with a scalloped appearance while the inner table is usually intact (4647).
Benign tumors or slow growing cysts can result in overlying skull thinning because of a chronic pressure effect. Smooth-margined calvarial thinning without periosteal reaction or new bone formation suggests that the underlying lesion is benign.
Parry Romberg syndrome is rare neurocutaneous disorder also known as progressive hemifacial atrophy. One side of the face, including the skin, subcutaneous tissue, muscles, cartilage, and underlying bony structures, becomes slowly and progressively atrophic. Radiologic findings include bone and soft-tissue atrophy of varying degrees, which do not cross the midline (48).
Calvarial lesions vary from clinically insignificant lesions to life-threatening lesions such as bone metastases. While bony lesions of the calvarium are easily identified using brain CTs, bone marrow lesions can be visualized better on brain MRIs than on brain CTs. Diffusion-weighted MRI is helpful in detection and diagnosis of bone marrow lesions. The categorical imaging approach presented here will be helpful in narrowing the list of possible diagnoses of calvarial lesions.
Figures and Tables
Fig. 1
A 70-year-old male with multiple myeloma.
On lateral skull radiograph (A) and axial CT (B), multiple punch-out osteolytic lesions in the skull (salt and pepper) are seen, a pathognomonic finding of multiple myeloma.
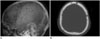
Fig. 2
A 70-year-old female with renal cell carcinoma.
A. On contrast-enhanced CT, two huge osteolytic soft tissue masses with extreme enhancement in right parietooccipital and left frontotemporal bone are seen (arrows).
B. On lateral skull radiograph, two huge osteolytic lesions (arrows) are seen.
C. On MRI, huge soft tissue metastatic tumors show heterogeneous signal intensity on diffusion-weighted imaging (arrows).
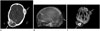
Fig. 3
A 4-year-old girl with Langerhans cell histiocytosis.
A. On lateral skull radiograph, a focal osteolytic lesion (arrow) is visible.
B. Non-contrast CT shows a localized osteolytic lesion with beveled edges in the right frontal bone (arrow).
C, D. On MRI, the osteolytic mass shows heterogeneous signal intensity on a T2-weighted image (arrow) (C) and marked enhancement with focal cystic change on the gadolinium-enhanced T1-weighted image (arrow) (D).

Fig. 4
A 45-year-old male with osseous hemangioma.
A. On lateral skull radiograph, a small osteolytic lesion (arrow) is seen in the right parietal skull.
B, C. A focal mass is detected with non-enhancing low signal intensity (arrow) on coronal gadolinium-enhanced T1-weighted MR image (B) and high signal intensity (arrow) on coronal T2-weighted MR image (C).
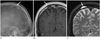
Fig. 5
A 13-year-old boy with epidermoid cyst of the skull.
Lateral skull radiograph (A) and axial CT (B) show a large lucent area with well-defined sclerotic margin (arrow) in the right parietal bone. The lesion shows heterogeneous bright signal intensity on coronal T2-weighted image (arrow) (C), and shows thin wall enhancement on non-contrast (arrow) (left in D) and contrast-enhanced T1-weighted images (arrow) (right in E).

Fig. 6
A 58-year-old female with intracalvarial meningioma.
A. A small well-defined radiolucent lesion (arrow) is seen on lateral skull radiograph.
B. The pre-contrast CT scan shows a well-defined mildly expansile osteolytic meningioma (arrow) on the right parietal bone.
C, D. This mass shows iso-signal intensity (C) on T2-weighted image (arrow) and contrast enhancement (arrow) (D) when gadolinium is applied.

Fig. 7
A 35-year-old male with fibrous dysplasia.
A. On non-contrast CT, an expansile lesion (arrow) with internal ground-glass opacity emerging from the occipital bone is seen.
B, C. On coronal T1-weighted image (B) and T2-weighted image (C), the lesion shows iso signal intensity (arrow) and mixed signal intensity (arrow), respectively.
D. It shows heterogeneous but marked enhancement (arrow) when gadolinium is added.

Fig. 8
A 59-year-old male with skull lymphoma.
A. On axial gadolinium-enhanced T1-weighted MR images, multiple homogeneous enhancing mass lesions are seen in both parietal bones (arrow in right parietal bone lesion).
B, C. The lesions show hyperintensity on diffusion-weighted imaging (arrow) (B) and iso signal intensity on the T2-weighted image (arrow) (C).
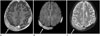
Fig. 9
A 1-year-old girl with desmoplastic fibroma.
A. A focal osteolytic lesion with sclerotic border (arrow) is seen near the right coronal suture on non-contrast CT.
B, C. The lesion shows prominent enhancement on the gadolinium-enhanced T1-weighted image (arrow) (B), but shows low signal intensity relative to brain parenchyma on diffusion-weighted imaging (arrow) (C).
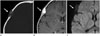
Fig. 10
A 45-year-old man with atretic cephalocele and persistent falcine sinus.
Coronal plain radiograph (A) shows a well-defined bony defect (arrow) in the interparietal region. On a T2-weighted image (B), a U-shaped hypointense subscalp lesion (arrows) which extends to a congenital bony defect is detected. Anomalous persistent falcine sinus is noted as well (open arrow). On contrast-enhanced MR venograph, marked stenosis (arrow) of the persistent falcine sinus (open arrow) at the junction of the falcine and superior sagittal sinuses is noted (C). Reprinted from Cho J, Kim MY, Roh HG, Moon WJ. MR images of spontaneously involuted atretic cephalocele concomitant with persistent falcine sinus in an adult. J Korean Soc Magn Reson Med 2006;10:117-120; with permission.
Table 1
Calvarial Lesions from Our Institution Database (n = 256)

Table 2
Categories and Pathologies Based on Involvement Pattern of Calvarial Lesions

Acknowledgments
This study was supported by Konkuk University in 2015 and by a grant of the Korea Healthcare technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI12C0713).
References
1. Lloret I, Server A, Taksdal I. Calvarial lesions: a radiological approach to diagnosis. Acta Radiol. 2009; 50:531–542.
2. Garfinkle J, Melançon D, Cortes M, Tampieri D. Imaging pattern of calvarial lesions in adults. Skeletal Radiol. 2011; 40:1261–1273.
3. Moon WJ, Lee MH, Chung EC. Diffusion-weighted imaging with sensitivity encoding (SENSE) for detecting cranial bone marrow metastases: comparison with T1-weighted images. Korean J Radiol. 2007; 8:185–191.
4. Sommer G, Klarhöfer M, Lenz C, Scheffler K, Bongartz G, Winter L. Signal characteristics of focal bone marrow lesions in patients with multiple myeloma using whole body T1w-TSE, T2w-STIR and diffusion-weighted imaging with background suppression. Eur Radiol. 2011; 21:857–862.
5. Prayer D, Grois N, Prosch H, Gadner H, Barkovich AJ. MR imaging presentation of intracranial disease associated with Langerhans cell histiocytosis. AJNR Am J Neuroradiol. 2004; 25:880–891.
6. Willatt JM, Quaghebeur G. Calvarial masses of infants and children. A radiological approach. Clin Radiol. 2004; 59:474–486.
7. Kilborn TN, Teh J, Goodman TR. Paediatric manifestations of Langerhans cell histiocytosis: a review of the clinical and radiological findings. Clin Radiol. 2003; 58:269–278.
8. Bastug D, Ortiz O, Schochet SS. Hemangiomas in the calvaria: imaging findings. AJR Am J Roentgenol. 1995; 164:683–687.
9. Amaral L, Chiurciu M, Almeida JR, Ferreira NF, Mendonça R, Lima SS. MR imaging for evaluation of lesions of the cranial vault: a pictorial essay. Arq Neuropsiquiatr. 2003; 61:521–532.
10. Azar-Kia B, Sarwar M, Marc JA, Schechter MM. Intraosseous meningioma. Neuroradiology. 1974; 6:246–253.
11. Lang FF, Macdonald OK, Fuller GN, DeMonte F. Primary extradural meningiomas: a report on nine cases and review of the literature from the era of computerized tomography scanning. J Neurosurg. 2000; 93:940–950.
12. Crawford TS, Kleinschmidt-DeMasters BK, Lillehei KO. Primary intraosseous meningioma. Case report. J Neurosurg. 1995; 83:912–915.
13. Greenspan A, Jundt G, Remagen W. Differential Diagnosis in Orthopaedic Oncology. 2nd ed. Philadelphia: Linppincott Williams & Wilkins;2006.
14. Lisle DA, Monsour PA, Maskiell CD. Imaging of craniofacial fibrous dysplasia. J Med Imaging Radiat Oncol. 2008; 52:325–332.
15. Ortiz O, Schochet S, Bastug D. Imaging evaluation and clinicopathologic correlation of mass lesions involving the calvaria Part II: tumoral and inflammatory lesions. Int J Neuroradiol. 1999; 5:151–165.
16. Lo CP, Chen CY, Chin SC, Juan CJ, Hsueh CJ, Chen A. Disappearing calvarium in Gorham disease: MR imaging characteristics with pathologic correlation. AJNR Am J Neuroradiol. 2004; 25:415–418.
17. Agrawal A, Sinha A. Lymphoma of frontotemporal region with massive bone destruction and intracranial and intraorbital extension. J Cancer Res Ther. 2008; 4:203–205.
18. Kantarci M, Erdem T, Alper F, Gundogdu C, Okur A, Aktas A. Imaging characteristics of diffuse primary cutaneous B-cell lymphoma of the cranial vault with orbital and brain invasion. AJNR Am J Neuroradiol. 2003; 24:1324–1326.
19. Rabin D, Ang LC, Megyesi J, Lee DH, Duggal N. Desmoplastic fibroma of the cranium: case report and review of the literature. Neurosurgery. 2003; 52:950–954. discussion 954.
20. Kumar R, Madewell JE, Lindell MM, Swischuk LE. Fibrous lesions of bones. Radiographics. 1990; 10:237–256.
21. Cho J, Kim MY, Roh HG, Moon WJ. MR images of spontaneously involuted atretic cephalocele concomitant with persistent falcine sinus in an adult. J Korean Soc Magn Reson Med. 2006; 10:117–120.
22. Sinclair AG, Scoffings DJ. Imaging of the post-operative cranium. Radiographics. 2010; 30:461–482.
23. Kanazawa R, Yoshida D, Takahashi H, Matsumoto K, Teramoto A. Osteosarcoma arising from the skull--case report. Neurol Med Chir (Tokyo). 2003; 43:88–91.
24. Bose B. Primary osteogenic sarcoma of the skull. Surg Neurol. 2002; 58:234–239. discussion 239-240.
25. Anslow P. Cranial bacterial infection. Eur Radiol. 2004; 14:Suppl 3. E145–E154.
26. Esposito G, Della Pepa GM, Sturiale CL, Gaudino S, Anile C, Pompucci A. Hypertrophic arachnoid granulation of the occipital bone: neuroradiological differential diagnosis. Clin Neuroradiol. 2011; 21:239–243.
27. Branan R, Wilson CB. Arachnoid granulations simulating osteolytic lesions of the calvarium. AJR Am J Roentgenol. 1976; 127:523–525.
28. Fortman BJ, Kuszyk BS, Urban BA, Fishman EK. Neurofibromatosis type 1: a diagnostic mimicker at CT. Radiographics. 2001; 21:601–612.
29. Nemeth AJ, Henson JW, Mullins ME, Gonzalez RG, Schaefer PW. Improved detection of skull metastasis with diffusion-weighted MR imaging. AJNR Am J Neuroradiol. 2007; 28:1088–1092.
30. Cerase A, Priolo F. Skeletal benign bone-forming lesions. Eur J Radiol. 1998; 27:Suppl 1. S91–S97.
31. Greenspan A. Benign bone-forming lesions: osteoma, osteoid osteoma, and osteoblastoma. Clinical, imaging, pathologic, and differential considerations. Skeletal Radiol. 1993; 22:485–450.
32. Osborn AG. Osborn's Brain: Imaging, Pathology, and Anatomy. Salt Lake City: Amirsys Publishing, Inc.;2012.
33. Lucey BP, March GP Jr, Hutchins GM. Marked calvarial thickening and dural changes following chronic ventricular shunting for shaken baby syndrome. Arch Pathol Lab Med. 2003; 127:94–97.
34. Chatterjee S. Hyperpigmentation associated with minocycline therapy. CMAJ. 2007; 176:321–322.
35. Kattan KR. Calvarial thickening after Dilantin medication. Am J Roentgenol Radium Ther Nucl Med. 1970; 110:102–105.
36. Tehranzadeh J, Fung Y, Donohue M, Anavim A, Pribram HW. Computed tomography of Paget disease of the skull versus fibrous dysplasia. Skeletal Radiol. 1998; 27:664–672.
37. Smith SE, Murphey MD, Motamedi K, Mulligan ME, Resnik CS, Gannon FH. From the archives of the AFIP. Radiologic spectrum of Paget disease of bone and its complications with pathologic correlation. Radiographics. 2002; 22:1191–1121.
38. Hollar MA. The hair-on-end sign. Radiology. 2001; 221:347–348.
39. Uemura A, O'uchi T, Kikuchi Y. Double skull sign of ossified cephalohematoma on CT. Eur J Radiol Extra. 2004; 49:35–36.
40. Smith S, Hemphill RE. Hyperostosis frontalis interna. J Neurol Neurosurg Psychiatry. 1956; 19:42–45.
41. She R, Szakacs J. Hyperostosis frontalis interna: case report and review of literature. Ann Clin Lab Sci. 2004; 34:206–208.
42. Mahomed N, Sewchuran T, Mahomed Z. The copper-beaten skull. SA J Radiol. 2012; 16:25–26.
43. Glass RB, Fernbach SK, Norton KI, Choi PS, Naidich TP. The infant skull: a vault of information. Radiographics. 2004; 24:507–522.
44. Raja RA, Khemani VD, Sheikh S, Khan H. Craniosynostosis: early recognition prevents fatal complications. J Ayub Med Coll Abbottabad. 2011; 23:140–143.
45. Aviv RI, Rodger E, Hall CM. Craniosynostosis. Clin Radiol. 2002; 57:93–102.
46. Yiu Luk S, Fai Shum JS, Wai Chan JK, San Khoo JL. Bilateral thinning of the parietal bones: a case report and review of radiological features. Pan Afr Med J. 2010; 4:7.
47. Cederlund CG, Andrén L, Olivecrona H. Progressive bilateral thinning of the parietal bones. Skeletal Radiol. 1982; 8:29–33.
48. Moon WJ, Kim HJ, Roh HG, Oh J, Han SH. Diffusion tensor imaging and fiber tractography in Parry-Romberg syndrome. AJNR Am J Neuroradiol. 2008; 29:714–715.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download