Abstract
The outbreak of Middle East Respiratory Syndrome Corona Virus (MERS-CoV) infection in South Korea originated from Saudi Arabia. This virus shows high infectivity, and causes outbreaks of severe febrile respiratory infections in health care-associated settings. Herein, we reported a fatal case of MERS-CoV infection with a focus on the pulmonary radiologic findings. The initial chest computed tomography and radiographs of our patient showed ground-glass opacity in patchy distribution, followed by rapid progression of consolidation and pleural effusion in serial studies.
A major outbreak of the Middle East Respiratory Syndrome Corona Virus (MERS-CoV) was first reported in Riyadh, Saudi Arabia from 2012 to 2014 (1). The computed tomography (CT) images of patients in Saudi Arabia predominantly showed ground-glass opacity, mostly in the lower peripheral lobes of the lungs (2).
On May 20, 2015, the first case of MERS-CoV infection was reported in South Korea. A subsequent major outbreak in health care settings caused the disease to spread nationwide. Concerns over this outbreak were heightened, owing to a novel MERS-CoV with the potential to rapidly disseminate from person to person and devastate the lungs. Herein, we described the radiologic appearance of a fatal case of a novel MERS-CoV infection. The Yeouido St. Mary's Hospital Institutional Review Board (IRB) determined that our case report was exempt from IRB review on June 30, 2015.
A 71-year-old man presenting with a 4-day history of fever and dyspnea was admitted to our institution's emergency room. The patient had no recent history of traveling abroad. However, 11 days prior, he was hospitalized for 3 days in the same ward as the first patient diagnosed with MERS-CoV infection in South Korea. The 71-year-old patient had a medical history of a right nephrectomy, because of renal cancer, but his blood urea nitrogen and creatinine values were within normal limits. On physical examination, he had a blood pressure of 132/60 mm Hg, a heart rate of 96 beats/min, and a body temperature of 38.5℃. His arterial blood gas analysis in the room air environment revealed the following: pH, 7.526; PaCO2, 24.2 mm Hg; PaO2, 43.9 mm Hg; and oxygen saturation, 83.3%. The results of the initial laboratory evaluation were as follows: white blood cell count, 3010/mm3 (neutrophils, 74.4%); platelet count, 160000/mm3; and creatinine phosphate level, 36.75 mg/L (normal range, 0.0–5.0 mg/L). Based on the initial evaluations, the patient was diagnosed with systemic inflammatory response syndrome.
A chest radiograph (Fig. 1A) taken at another hospital showed an increased patchy opacity in the right upper lung field, and diffuse emphysematous changes in both lungs. A chest CT image (Fig. 1B, C) taken at another hospital showed a patchy ground-glass opacity in the subpleural portion of the right upper lobe and the apex of the left upper lobe, combined with interlobular septal thickening and a minimal amount of pleural effusion in both lungs. In addition to diffuse emphysematous changes in both lungs, multiple enlarged lymph nodes were noted in the hilar region and mediastinum. A subsequent chest radiograph and CT image (Fig. 1D-F) were obtained due to rapid progression of the patient's clinical condition. The follow-up CT examinations performed two days after those at the other hospital indicated that the extent of consolidation and ground-glass opacity in the right upper lobe had markedly increased and multifocal nodular consolidation had developed in both lower lobes. The amount of pleural effusion in both lungs was increased.
The real-time reverse transcriptase polymerase chain reaction (PCR) test using a sputum specimen was positive for MERS-CoV. The blood culture and examinations to detect co-pathogens including the respiratory virus multiplex PCR yielded negative results. Therefore, the patient was diagnosed as having a laboratory-confirmed MERS-CoV pneumonia without co-infection.
Consecutive chest radiographs on the following day revealed progression of the multifocal consolidations in the lower lung fields (Fig. 1G); in addition, the arterial blood gas analysis while maintaining the patient on 4 L of oxygen via mask, showed the same findings, as follows: pH, 7.510; PaCO2, 27.1 mm Hg; PaO2, 73.0 mm Hg; and oxygen saturation, 92.5%. The blood urea nitrogen and creatinine values were within normal limits. The patient was transferred to a country-designated isolation hospital, where he was admitted in a specialized intensive care unit with maximal precautions, including a negative pressure room. He died because of respiratory and renal failure on June 1, 2015, 11 days after symptom onset.
In April 2012, infections with MERS-CoV appeared throughout the Arabian Peninsula, primarily in Saudi Arabia (1). As of June 26, 2015, 1356 laboratory-confirmed cases of human infection with MERS-CoV have been reported to the World Health Organization (3). The clinical manifestations of MERS-CoV include fever (98%), cough (83%), and dyspnea (72%). Other common symptoms are myalgia, abdominal pain, nausea, vomiting, and diarrhea (4). The clinical spectrum varies from asymptomatic infection to rapidly progressive multi-organ failure and death. The general mortality rate is approximately 36% (5). This fatality rate increases with increasing age and the presence of any underlying comorbid medical disorders (4).
The basic reproduction number of MERS-CoV is about 0.60–0.69 and the estimated reproduction number during the outbreak in Saudi Arabia in the spring of 2014 was suggested as 3.5–6.7 in Jeddah, and 2.0–2.8 in Riyadh (46). On May 20, 2015, a person who had recently traveled to Saudi Arabia, Qatar, United Arab Emirates, and Bahrain was diagnosed positive for MERS-CoV infection in South Korea. This patient transmitted MERS-CoV to 29 additional laboratory-confirmed cases (7). Epidemiologic analysis is necessary due to the unusual pattern of transmission in South Korea, and the higher than established reproduction number. Two months epidemiologic investigation since May 20, 2015 revealed that a total of 186 patients were diagnosed in South Korea, among which, 36 mortality cases were reported (3).
The radiologic images of the pulmonary complications in MERS-CoV infection have the atypical pattern of a viral pneumonia. Ajlan et al. (8) reported that the common CT finding of MERS-CoV infection-related pneumonia is the predominance of an air-space opacity in the subpleural and basilar lung regions bilaterally. In this study, a ground-glass opacity was the earliest CT finding (2–5 days), followed by a ground-glass opacity and consolidation in combination, pleural effusion, and interlobular septal thickening. Peribronchovascular involvement may also occur and occasionally progress to a fibrotic process, resulting in an organized pneumonia in some patients; while cavitation and a tree-in-bud pattern are rare.
Das et al. (2) reported pleural effusion in all fatal cases, and thus, a correlation with poor prognosis and short-term mortality. In addition, mortality cases showed a higher proportion of recurrently fluctuating or consistently progressing radiographic deterioration of chest radiographs, and a greater number of involved lung segments in chest CT, as compared to patients who recovered.
The major finding of initial chest radiography and CT in our case was the presence of a subpleural ground-glass opacity in the right upper lung field. Subsequent chest radiography and CT showed rapid progression of the ground-glass opacity to confluent consolidation. This is reportedly the most common finding of MERS-CoV pneumonia (8). As in our case, progressive consolidative deterioration is associated with high mortality. The disease progresses until the lungs become completely consolidated or the subject dies (9). Furthermore, a small amount of pleural effusion that typically appears in the severe types of MERS-CoV pneumonia, was detected in both lungs (2). This patient is the second fatal case of MERS-CoV infection in South Korea.
The radiographic appearance of peripheral ground-glass opacity with a rapidly progressing confluent consolidation is not specific for MERS-CoV pneumonia. These findings are indistinguishable from other causes of atypical pneumonia and overlap with other types of viral pneumonia, such as H1N1 (novel swine-origin influenza A) and severe acute respiratory syndrome (2910). Therefore, the clinical manifestation is an integral part of obtaining an accurate diagnosis. Characteristic clinical features that include high fever, cough, dyspnea, chills, and myalgia and laboratory findings such as leukopenia and thrombocytopenia, in patients with recent exposure, is suggestive of MERS-CoV infection.
In conclusion, we reported a fatal case of MERS-CoV pneumonia in South Korea, focusing on the radiologic findings and chest CT findings. Recognizing the common radiologic findings of MERS-CoV pneumonia, and prognostic factors such as the presence of pleural effusion or confluent consolidation, would facilitate proper management of patients with suspected MERS-CoV infection.
Figures and Tables
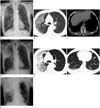 | Fig. 1A 71-year-old man diagnosed with Middle East Respiratory Syndrome Corona Virus.
A. The initial chest radiograph shows an ill-defined increased opacity in the right upper lung field.
B, C. Chest CT scans obtained on the same day as (A) demonstrate patchy ground-glass opacity and interlobular septal thickenings in the subpleural portion of right upper lobe (B). A small amount of bilateral pleural effusion is also noted (C).
D. Follow-up chest radiographs obtained 2 days later reveal progression of consolidation in right upper lung field and newly developed, ill-defined, increased opacity in both lower lung fields.
E, F. Follow-up chest CT scans obtained on the same day as (D) show markedly increased extent of consolidation and ground-glass opacity in the right upper lobe (E). Newly developed multifocal nodules in both lower lobes and increased amount of bilateral pleural effusion (F).
G. A further follow-up chest radiograph 2 days later, shows aggravated multifocal consolidations in the right upper lung field and both lower lung fields.
|
References
1. World Health Organization. Background and summary of novel coronavirus infection - as of 30 November 2012. Accessed Jan 18, 2016. Available at: http://www.who.int/csr/disease/coronavirus_infections/update_20121130/en/.
2. Das KM, Lee EY, Enani MA, AlJawder SE, Singh R, Bashir S, et al. CT correlation with outcomes in 15 patients with acute Middle East respiratory syndrome coronavirus. AJR Am J Roentgenol. 2015; 204:736–742.
3. World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV) - Republic of Korea. Accessed Jan 18, 2016. Available at: http://www.who.int/csr/don/26-june-2015-merskorea/en/.
4. Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013; 13:752–761.
5. World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV). Accessed Jan 18, 2016. Available at: http://www.who.int/mediacentre/factsheets/mers-cov/en/.
6. Majumder MS, Rivers C, Lofgren E, Fisman D. Estimation of MERS-coronavirus reproductive number and case fatality rate for the Spring 2014 Saudi Arabia outbreak: insights from publicly available Data. PLoS Curr. 2014; 12. 18. [Epub]. DOI: 10.1371/currents.outbreaks.98d2f8f3382d84f390736cd5f5fe133c.
7. World Health Organization. Summary and risk assessment of current situation in Republic of Korea and China. Accessed Jan 18, 2016. Available at: http://www.who.int/csr/disease/coronavirus_infections/risk-assessment-3june2015/en/.
8. Ajlan AM, Ahyad RA, Jamjoom LG, Alharthy A, Madani TA. Middle East respiratory syndrome coronavirus (MERS-CoV) infection: chest CT findings. AJR Am J Roentgenol. 2014; 203:782–787.
9. Wong KT, Antonio GE, Hui DS, Lee N, Yuen EH, Wu A, et al. Severe acute respiratory syndrome: radiographic appearances and pattern of progression in 138 patients. Radiology. 2003; 228:401–406.
10. Agarwal PP, Cinti S, Kazerooni EA. Chest radiographic and CT findings in novel swine-origin influenza A (H1N1) virus (S-OIV) infection. AJR Am J Roentgenol. 2009; 193:1488–1493.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download