Abstract
Purpose
The purpose of this study was to identify common underlying etiologies that may be responsible for isolated acute nontraumatic cortical subarachnoid hemorrhage (cSAH) by analysis of magnetic resonance imaging (MRI) findings of the brain.
Materials and Methods
From August 2005 to February 2014, 15 cSAH patients were admitted to our institution. All patients with cSAH underwent brain MRI and magnetic resonance angiography as a part of their initial evaluation. An analysis of the patients' medical history, clinical presentations, and brain MRI findings was retrospectively performed.
Results
Among the combined pathologies that were suspected causes of cSAH, 11 patients showed acute or subacute cerebral infarctions at the ipsilateral hemisphere of cSAH on the diffusion-weighted images. Four of 11 patients had only cerebral infarction, but the other 7 had combined vasculopathy of extra- and intracranial arteries. Four of 15 patients who did not have cerebral infarction, had intracranial artery stenosis, or showed possible cerebral amyloid angiopathy, or no abnormal findings on the brain MRI.
Isolated acute nontraumatic cortical subarachnoid hemorrhages (cSAHs), also called convexal subarachnoid hemorrhages and convexity subarachnoid hemorrhages, are rare cases of bleeding in the subarachnoid space. They are localized to the convexities of the brain, usually several cortical sulci, without involving the brain parenchyma or extending into the interhemispheric fissures, basal cisterns, or ventricles. Unlike conventional subarachnoid hemorrhages (SAHs), rupture of the intracranial arterial aneurysm may not induce a cSAH. Various etiologies are described, such as cerebral venous thrombosis, posterior reversible leukoencephalopathy syndrome (PRES), reversible cerebral vasoconstriction syndrome (RCVS), coagulopathy, vasculitidis, vascular malformations such as pial arteriovenous malformations, dural arteriovenous fistulas, cavernomas, cerebral amyloid angiopathy (CAA), brain abscesses, atherosclerotic carotid disease, and primary and secondary brain neoplasms (1234567891011121314). Kumar et al. (1) suggested two main etiologic groups including cSAH patients < 60 years old presenting with thunderclap headaches that are ascribed to RCVS; and, cSAH patients > 60 years old presenting with transient focal symptoms and frequently exhibiting features suggestive of CAA under imaging methods (114). However, the ischemic stroke, especially the cerebral infarction or vasculopathy of extra- and intracranial arteries, such as high-grade stenosis, is not considered a common etiology of cSAHs.
We analyzed the associated radiologic findings of magnetic resonance imaging (MRI) of the brains of cSAH patients at a single center, in order to emphasize the importance of ischemic strokes as a common cause of cSAH.
Our hospital's Institutional Review Board approved this retrospective study.
We reviewed medical records, including hospital notes, laboratory data, and imaging studies, to exclude traumatic SAH and patients who exhibited the spreading of blood into Sylvian or interhemispheric fissures, as well as those with basal cisterns. We also excluded patients with intracerebral bleeding that may have ruptured into the subarachnoid space, patients with damage to the brain parenchyma adjacent to the cSAH, and patients with a large degree of cerebral infarction adjacent to the cSAH. Patients with only superficial siderosis were also excluded from the study. Moreover, patients with preceding episodes of head trauma, intracranial aneurysms, or thrombolytic therapy were excluded.
We retrospectively searched the radiologic reports of all the patients identified with cSAHs by initial brain CT scans or MRI at a single institution. From August 2005 to February 2014, fifteen cSAH patients were admitted to our institution including seven males and eight females with an average age of 47 years (range: 30 to 79 years). The patients who exhibited cSAH in the initial brain CT imaging underwent brain MRI for the further evaluation of combined pathologies. Thus, all patients with cSAH underwent brain MRI and magnetic resonance angiography (MRA). A retrospective analysis was conducted on the patients' medical history, clinical presentations, and brain MRI findings. Three (20%) patients had diabetes mellitus (DM), three (20%) patients had hypertension (HTN), and one (7%) had both. One (7%) patient had atrial fibrillation. One (7%) had chronic kidney disease and double primary cancers of the lung and prostate. One (7%) had previous myocardial infarction (Table 1). All the patients had a normal platelet count, prothrombin time, and activated partial thromboplastin time.
Brain MRI and MRA systems operating at 1.5T (Signa Excite; GE Healthcare, Milwaukee, WI, USA) and 3.0T (Discovery MR 750; GE Healthcare) were available. The brain MRI included an axial fluid-attenuated inversion recovery (FLAIR) image, a diffusion- weighted image (DWI), a contrast-enhanced T1-weighted image (T1WI), and a gradient echo (GRE) T2*-weighted sequence. The MRA was performed using the time-of-flight method.
The contrast-enhanced T1WI was obtained by intravenous injection of 20 cc Meglumine Gadoterate (Dotarem; Guerbet, Paris, France) at 3 mL/s in each patient.
A brain dynamic susceptibility contrast perfusion study using MRI was performed in eleven cases. Perfusion maps showing the cerebral blood volume, cerebral blood flow, mean transit time, and time to peak were obtained by post-processing.
Among the fifteen cases involving cSAHs, eight (53%) involved cSAHs above the frontal cortex, and three (20%) involved cSAHs along the central sulcus. Three other cases (20%) exhibited cSAHs in the parietal area, and in one case (7%), bleeding occurred in the temporal area. All cSAHs were observed in the FLAIR images. The GRE images also adequately represented cSAHs.
The patients experienced a variety of symptoms. Primary motor or sensory change was regarded as the most common symptom. Nine (60%) patients exhibited hemiplegia, involuntary movement, or transient sensory loss. The latter involved the loss of consciousness, which occurred in two (13%) patients. Two (13%) patients complained of headache, and one (7%) reported dizziness.
Regarding the combined pathologies of the cSAH, eleven (73%) patients exhibited acute cerebral infarctions in the ipsilateral hemisphere on the DWIs. Four of the eleven patients had an acute cerebral infarction without extra- or intracranial vasculopathy. Vasculopathy of the intracranial arteries was observed in five of the eleven patients with cerebral infarction; of the five patients, three had stenosis of the intracranial arteries, and one had the occlusion of the right middle cerebral artery (MCA) M1 portion, and one had a dissection of the left anterior cerebral artery A2 portion. The remaining two patients with acute cerebral infarction had the stenosis of the extracranial arteries.
Two (13%) patients exhibited stenosis of the intracranial artery that supplied blood flow to the vascular territory containing the cSAH, without evidence of cerebral infarction. Both had the stenosis of the left MCA.
Figs. 1 to 4 showed cases of typical cSAH associated with an ischemic stroke, such as acute cerebral infarction and the highgrade stenosis of extra- or intracranial arteries.
Only one (7%) patient showed multiple intracerebral hemorrhage (ICH), which satisfied the Boston criteria for probable CAA (16). The remaining patient (7%) did not have any combined radiological abnormalities and exhibited only a cSAH. Table 2 presented the concise results.
Eleven patients who underwent brain dynamic susceptibility contrast perfusion study employing MRI showed no perfusion defect; however, three of the eleven patients exhibited the hyperperfusion state of the infarcted area. This could be considered a "luxury perfusion" after the acute cerebral infarction.
cSAH is rarely considered as a type of nonaneurysmal subarachnoid bleeding due to its varied etiologies. The etiologies of cSAH are under ongoing debate. Kumar et al. (1) suggested that RCVS and the CAA could be the major etiologies of cSAH. Beitzke et al. (15) also reported that RCVS is a major etiology of the cSAH in patients ≤ 60 years old, but RCVS could not be confirmed in these patients via angiograms. The exact pathophysiologic mechanism of cSAHs in patients with RCVS is unclear; however, there is speculation that it arises from abrupt changes in the cerebral arterial tone (1718). Patients > 60 years have an increased association of cSAHs with the CAA (15). Spitzer et al. (19) and Refai et al. (20) reported that PRES is the major underlying etiology of cSAH.
In contrast to these previous reports, our study showed that the ischemic stroke is the major etiology of the cSAH. Ischemic stroke, including cerebral infarctions, extra- or intracranial arterial stenosis, and intracranial arterial dissections was the confirmed cause in thirteen of fifteen cases (87%). Among the combined ischemic stroke disease entities, cerebral infarction was the most common in the cSAH patients. Some cases with cerebral infarction involved a combination of underlying stenosis and the dissection of the intracranial arteries.
Three letters to the editor reported cases similar to those considered in our study. Geraldes et al. (21) reported two cases of cSAH associated with acute carotid artery occlusion on the unilateral side; Kleinig et al. (22) described three cases of cSAH with bilateral internal carotid artery (ICA) stenosis; and Chandra et al. (23) also described a cSAH case with severe ICA stenosis. These authors could not explain the underlying mechanism but proposed that the acute alteration of the hemodynamic stress may damage the already maximally dilated pial collateral vasculature, leading to cSAHs. They proposed that systemic disturbances such as a sudden rise in blood pressure or a coagulation disorder in the presence of pre-damaged vessels account for these coincidental observations (212223). Some of our cases, particularly those of intracranial arterial stenosis without cerebral infarction, supported this hypothesis.
A possible underlying mechanism of cSAHs is as follows. Changes in the blood-brain barrier (BBB) reportedly occur in an acute or chronic ischemic insult (24252627282930). BBB abnormalities are reported in patients with small-vessel diseases secondary to HTN and DM (28). Particularly for lacunar strokes, BBB dysfunction was confirmed as a part of the pathogenesis (29). The disruption of the BBB during ischemia, hypoxia, or a hemorrhage progresses by two steps. The initial opening of the BBB is reversible and associated with the activation of matrix metalloproteinases. The second opening of the BBB occurs 24 to 48 h after a reperfusion, depending on the length of the ischemia. The BBB is damaged after these two steps, and its permeability increases (28). The qualitative visualization of Gadolinium-diethylenetriaminepenta- acetic acid (Gd-DTPA) indirectly indicates the leakiness of the blood vessels in the acute stage of a stroke. Several hours after the injection of Gd-DTPA, FLAIR images exhibit the disruption of the BBB, which appears as an enhancement in the sulci over the infarcted area in 1/3rd of acute ischemic stroke patients (31). BBB damage is an important factor in the growth of ICHs and hemorrhagic transformations (303233). The permeability of the BBB can increase in acute strokes because of the breakdown of the BBB by the ischemic insult. The BBB breakdown can potentially explain the mechanism underlying the damage to the cerebral vasculature in largeartery stenosis, which causes a chronic ischemic insult to the brain. Therefore, the leakage of blood through the BBB to the subarachnoid space in patients with an ischemic stroke may be possible.
In contrast to many previous studies, our study showed that ischemic stroke is the major etiology of the cSAH. The possible reason for the current results is that the difference in proportionate distribution of ischemic-stroke subtypes between Western and Korean populations. Most previous studies on cSAHs are conducted on Western patients. Furthermore, Western patients have a significantly greater proportion of cardioembolic and cryptogenic stroke, as compared with Koreans (34). The Korean population has a high incidence of cardioembolic stroke but also exhibits the greatest proportion of large-artery atherosclerosis among the ischemic stroke subtypes (35). The higher relative incidence of large-artery atherosclerosis in Korean patients may be one of the reasons why ischemic strokes are frequently associated with cSAHs. Thus, large-artery atherosclerosis may cause a chronic ischemic insult to the brain, which could be the mechanism underlying cSAHs.
Our study had some limitations. Our observations reflect experiences at a single tertiary care center, as obtained by a retrospective analysis. Thus, the study may have sources of selection bias.
In conclusion, ischemic stroke could be a common underlying etiology of isolated acute nontraumatic cSAHs. Damage to the cerebral vasculature, particularly the breakdown of the BBB due to an ischemic stroke, is the possible and dominant mechanism underlying the cSAH.
Figures and Tables
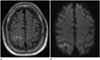 | Fig. 174-year-old man with isolated acute nontraumatic cortical subarachnoid hemorrhage (cSAH) and acute cerebral infarction.
A. FLAIR image shows a cSAH in the right central sulcus (arrowheads). The adjacent right parietal cortex exhibits a hyperintense signal in this image (black arrows).
B. DWI shows a patchy hyperintense signal in the same area, suggesting acute cerebral infarction (white arrows). The cSAH is represented by a hypointense signal along the right central sulcus on the DWI.
DWI = diffusion-weighted image, FLAIR = fluid-attenuated inversion recovery
|
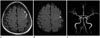 | Fig. 252-year-old woman with cSAH and acute cerebral infarction and intracranial arterial stenosis.
A. FLAIR image shows cSAH in the sulci of left frontal area.
B. DWI shows focal acute infarction in the left frontal cortical area (arrowhead).
C. Magnetic resonance angiographic (MRA) image shows the stenosis of the left middle cerebral artery M1 portion (arrow).
cSAH = cortical subarachnoid hemorrhage, DWI = diffusion-weighted image, FLAIR = fluid-attenuated inversion recovery
|
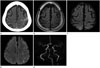 | Fig. 366-year-old man with cSAH and acute cerebral infarction and extracranial arterial stenosis.
A. Pre-enhanced brain CT image shows localized high density along left central sulcus, suggesting cSAH.
B. FLAIR image shows the localized high-signal intensity in the left central sulcus.
C, D. DWI (D) shows multiple small hyperintense foci in the left parietal cortex departing from the cSAH, suggesting acute cerebral infarction. The cSAH is represented by a hypointense signal along the right central sulcus on the DWI (C).
E. MRA image shows the stenosis of the petrous segment of the left internal carotid artery (arrow). cSAH = cortical subarachnoid hemorrhage, DWI = diffusion-weighted image, FLAIR = fluid-attenuated inversion recovery, MRA = magnetic resonance angiographic
|
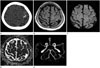 | Fig. 478-year-old woman with cSAH and intracranial arterial stenosis.
A. Pre-enhanced brain CT image shows a localized high density along the left central sulcus (arrowheads).
B. FLAIR image shows cSAH in the left central sulcus (white arrows).
C, D. DWI (C) shows a strong linear signal along the left central sulcus (the same area), indicating a cSAH (black arrows). However, the apparent diffusion coefficient map (D) shows no evidence of diffusion restriction; thus, there is no evidence of acute cerebral infarction.
E. However, the MRA image shows the stenosis of the left middle cerebral artery M1 portion (arrow).
cSAH = cortical subarachnoid hemorrhage, DWI = diffusion-weighted image, FLAIR = fluid-attenuated inversion recovery, MRA = magnetic resonance angiographic
|
Table 1
Detailed Overview of the Patients' Data

ACA = anterior cerebral artery, cSAH = cortical subarachnoid hemorrhage, DM = diabetes mellitus, HTN = hypertension, ICA = internal carotid artery, ICH = intracerebral hemorrhage, MCA = middle cerebral artery, MRA = magnetic resonance angiography, MRI = magnetic resonance imaging, N-S = non-specific
Table 2
Etiologies of Isolated Acute Nontraumatic cSAH Based on Brain MRI Findings

References
1. Kumar S, Goddeau RP Jr, Selim MH, Thomas A, Schlaug G, Alhazzani A, et al. Atraumatic convexal subarachnoid hemorrhage: clinical presentation, imaging patterns, and etiologies. Neurology. 2010; 74:893–899.
2. Cuvinciuc V, Viguier A, Calviere L, Raposo N, Larrue V, Cognard C, et al. Isolated acute nontraumatic cortical subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2010; 31:1355–1362.
3. Oppenheim C, Domigo V, Gauvrit JY, Lamy C, Mackowiak-Cordoliani MA, Pruvo JP, et al. Subarachnoid hemorrhage as the initial presentation of dural sinus thrombosis. AJNR Am J Neuroradiol. 2005; 26:614–617.
4. Benabu Y, Mark L, Daniel S, Glikstein R. Cerebral venous thrombosis presenting with subarachnoid hemorrhage. Case report and review. Am J Emerg Med. 2009; 27:96–106.
5. Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007; 130(Pt 12):3091–3101.
6. Kumar R, Wijdicks EF, Brown RD Jr, Parisi JE, Hammond CA. Isolated angiitis of the CNS presenting as subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1997; 62:649–651.
7. Jain R, Deveikis J, Hickenbottom S, Mukherji SK. Varicellazoster vasculitis presenting with intracranial hemorrhage. AJNR Am J Neuroradiol. 2003; 24:971–974.
8. Kannoth S, Iyer R, Thomas SV, Furtado SV, Rajesh BJ, Kesavadas C, et al. Intracranial infectious aneurysm: presentation, management and outcome. J Neurol Sci. 2007; 256:3–9.
9. Osanai T, Kuroda S, Nakayama N, Yamauchi T, Houkin K, Iwasaki Y. Moyamoya disease presenting with subarachnoid hemorrhage localized over the frontal cortex: case report. Surg Neurol. 2008; 69:197–200.
10. Shah AK. Non-aneurysmal primary subarachnoid hemorrhage in pregnancy-induced hypertension and eclampsia. Neurology. 2003; 61:117–120.
11. Karabatsou K, Lecky BR, Rainov NG, Broome JC, White RP. Cerebral amyloid angiopathy with symptomatic or occult subarachnoid haemorrhage. Eur Neurol. 2007; 57:103–105.
12. Vilela P, Saraiva P, Goulão A. Intracranial angiolipoma as cause of subarachnoid haemorrhage. Case report and review of the literature. Neuroradiology. 2005; 47:91–99.
13. Hentschel S, Toyota B. Intracranial malignant glioma presenting as subarachnoid hemorrhage. Can J Neurol Sci. 2003; 30:63–66.
14. Raposo N, Viguier A, Cuvinciuc V, Calviere L, Cognard C, Bonneville F, et al. Cortical subarachnoid haemorrhage in the elderly: a recurrent event probably related to cerebral amyloid angiopathy. Eur J Neurol. 2011; 18:597–603.
15. Beitzke M, Gattringer T, Enzinger C, Wagner G, Niederkorn K, Fazekas F. Clinical presentation, etiology, and long-term prognosis in patients with nontraumatic convexal subarachnoid hemorrhage. Stroke. 2011; 42:3055–3060.
16. Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001; 56:537–539.
17. Vinters HV, Gilbert JJ. Cerebral amyloid angiopathy: incidence and complications in the aging brain II The distribution of amyloid vascular changes. Stroke. 1983; 14:924–928.
18. Thal DR, Ghebremedhin E, Orantes M, Wiestler OD. Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol. 2003; 62:1287–1301.
19. Spitzer C, Mull M, Rohde V, Kosinski CM. Non-traumatic cortical subarachnoid haemorrhage: diagnostic work-up and aetiological background. Neuroradiology. 2005; 47:525–531.
20. Refai D, Botros JA, Strom RG, Derdeyn CP, Sharma A, Zipfel GJ. Spontaneous isolated convexity subarachnoid hemorrhage: presentation, radiological findings, differential diagnosis, and clinical course. J Neurosurg. 2008; 109:1034–1041.
21. Geraldes R, Santos C, Canhão P. Atraumatic localized convexity subarachnoid hemorrhage associated with acute carotid artery occlusion. Eur J Neurol. 2011; 18:e28–e29.
22. Kleinig TJ, Kimber TE, Thompson PD. Convexity subarachnoid haemorrhage associated with bilateral internal carotid artery stenoses. J Neurol. 2009; 256:669–671.
23. Chandra RV, Leslie-Mazwi TM, Oh D, Mehta B, Yoo AJ. Extracranial internal carotid artery stenosis as a cause of cortical subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2011; 32:E51–E52.
24. Jin X, Liu J, Yang Y, Liu KJ, Yang Y, Liu W. Spatiotemporal evolution of blood brain barrier damage and tissue infarction within the first 3h after ischemia onset. Neurobiol Dis. 2012; 48:309–316.
25. Ivens S, Gabriel S, Greenberg G, Friedman A, Shelef I. Bloodbrain barrier breakdown as a novel mechanism underlying cerebral hyperperfusion syndrome. J Neurol. 2010; 257:615–620.
26. Hjort N, Wu O, Ashkanian M, Sølling C, Mouridsen K, Christensen S, et al. MRI detection of early blood-brain barrier disruption: parenchymal enhancement predicts focal hemorrhagic transformation after thrombolysis. Stroke. 2008; 39:1025–1028.
27. Topakian R, Barrick TR, Howe FA, Markus HS. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry. 2010; 81:192–197.
28. Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011; 42:3323–3328.
29. Wardlaw JM, Farrall A, Armitage PA, Carpenter T, Chappell F, Doubal F, et al. Changes in background blood-brain barrier integrity between lacunar and cortical ischemic stroke subtypes. Stroke. 2008; 39:1327–1332.
30. Rosenberg GA. Neurological diseases in relation to the blood-brain barrier. J Cereb Blood Flow Metab. 2012; 32:1139–1151.
31. Henning EC, Latour LL, Warach S. Verification of enhancement of the CSF space, not parenchyma, in acute stroke patients with early blood-brain barrier disruption. J Cereb Blood Flow Metab. 2008; 28:882–886.
32. Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997; 28:1–5.
33. Montaner J, Alvarez-Sabín J, Molina CA, Anglés A, Abilleira S, Arenillas J, et al. Matrix metalloproteinase expression is related to hemorrhagic transformation after cardioembolic stroke. Stroke. 2001; 32:2762–2767.
34. White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005; 111:1327–1331.
35. Jung KH, Lee SH, Kim BJ, Yu KH, Hong KS, Lee BC, et al. Secular trends in ischemic stroke characteristics in a rapidly developed country: results from the Korean Stroke Registry Study (secular trends in Korean stroke). Circ Cardiovasc Qual Outcomes. 2012; 5:327–334.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download