Abstract
The subintimal arterial flossing with antegrade-retrograde intervention (SAFARI) technique is reportedly effective in severe peripheral vascular disease that cannot be treated with standard endovascular techniques including subintimal angioplasty. In this report, we used a target balloon with the Outback catheter to recanalize a thrombosed bypass graft that could not be treated successfully with SAFARI.
Subintimal recanalization is an established option for the treatment of chronic critical limb ischemia when other techniques fail or are unavailable (1). The primary limitation to successful subintimal recanalization is failure to re-enter the true lumen after subintimal crossing of the occlusion. Reentry of the true lumen distally from an antegrade access point is impossible in up to 20% of patients (2). In these cases, the subintimal arterial flossing with antegrade-retrograde intervention (SAFARI) technique or reentry devices are useful alternatives.
The SAFARI technique can be achieved via popliteal, dorsalis pedis, or posterior tibial approaches (345). However, the retrograde approach is occasionally limited by failure to reenter the true lumen especially in patients with prior bypass graft, due to scarring induced by the previous operation (6).
Previous studies have shown variable success rates of 50 to 100% for re-entry devices in the treatment of infra-inguinal arterial occlusive diseases (278).
In this report, we used a retrograde target balloon with an antegrade Outback catheter, in order to gain access to the patient's distal artery.
A 76-year-old male who underwent left-side fem-pop bypass surgery 3 years prior (referred to hereafter as 'previous bypass graft') presented with left calf pain. Diagnostic CT angiogram revealed total occlusion of the left fem-pop bypass graft. A stent was already inserted in the distal part of the bypass graft at another hospital. The patient underwent another fem-pop bypass (referred to hereafter as 'new bypass graft') that was connected to the distal popliteal artery just above the origin of the anterior tibial artery. Three days after the surgery, the patient complained of a cold sensation in the left foot and exhibited a weak pulse. Doppler sonography indicated occlusion of the new fem-pop graft. Thus, we considered endovascular recanalization of the thrombosed bypass graft.
After local anesthesia with lidocaine injection, an 8 Fr vascular sheath (Arrow International, Reading, PA, USA) was inserted in the left common femoral artery using an antegrade approach.
On diagnostic angiography, total occlusion of both fem-pop bypass grafts was noted. Neither the popliteal artery, nor the peroneotibial trunk was clearly observed. The posterior tibial artery reconstituted by collateral vessels was the only artery supplying the left foot (Fig. 1).
First, we tried to recanalize the previous fem-pop bypass graft.
Suction thrombectomy using an 8 Fr guiding catheter (Boston Scientific Corporation, Natick, MA, USA) was used for the thrombus in the graft and the stented segment, followed by balloon dilatation with a 6-mm × 15-cm balloon catheter (Boston Scientific Corporation) for the multiple stenoses in the stented segment. Next, we attempted to pass a wire through the popliteal and tibioperoneal trunk occlusion site using a Rubicon catheter (Boston Scientific Corporation) with V14 wire (Boston Scientific Corporation), but this attempt failed. We re-attempted with Astato 30 wire (Asahi, Burlington, MA, USA) without success (Fig. 2).
Since we failed to recanalize the previous fem-pop bypass graft, recanalization of the new fem-pop bypass graft was considered. Surgical cut-down was performed at the left common femoral artery puncture site. Then, surgical thrombectomy was performed with a 6 Fr Fogarty catheter (Edwards Lifesciences, Irvine, CA, USA) and aspiration thrombectomy followed with an 8 Fr guiding catheter for remnant thrombus. We attempted wire passage through the popliteal and tibioperoneal trunk occlusion site.
After 2 failed attempts to gain access to the occluded popliteal and tibioperoneal trunk, an antegrade and retrograde approach was considered. After local anesthesia with lidocaine injection, the ankle portion of the posterior tibial artery was punctured with a 21 G micropuncture needle (Cook Medical, Bloomington, IN, USA). A Rubicon catheter was inserted directly through the puncture site without using any sheath; and V14 wire was used to pass the popliteal and tibioperoneal trunk occlusion site. However, the retrograde intraluminal approach to the occlusion area also failed. During negotiation, the wire entered the extravascular space, probably through the scar tissue caused by the previous operation.
It appeared impossible to pass the wire through the occlusion area, hence, we inserted a 3-mm × 2-cm balloon catheter (Boston Scientific Corporation) through the retrograde access site of the posterior tibial artery into the extravascular space adjacent to the occluded tibioperoneal trunk over the guide wire. Extraluminal position of the balloon catheter was confirmed by irregular spread of contrast material into the soft tissue, which was injected through the tip of the balloon catheter.
Since the previous bypass graft had a straighter course with distal arteries than the new bypass graft, we introduced the Outback catheter (Lumend, Redwood City, CA, USA) by an antegrade approach through the previous graft. Subsequently, the Outback catheter was placed at the level of the balloon catheter that was inserted by a retrograde approach.
We then targeted the balloon catheter under fluoroscopic guidance (Fig. 3). This was successful upon the first pass, and a 300-cm 0.014 ATW wire (Cordis, Miami, FL, USA) was introduced through the Outback needle into the posterior tibial artery. First, we tried to pass a 3-mm-diameter balloon along the ATW wire, but this was impossible. So, a 1.5-mm × 2-cm balloon was used to dilate the occlusion site followed by 3-mm × 10-cm balloon dilatation. Despite balloon dilatation, successive recoiling was noted, so a 5-mm × 10-cm Viabahn stent (W. L. Gore & Associates, Flagstaff, AZ, USA) was inserted in the occluded area. Balloon dilatation was performed with a 4-mm × 2-cm balloon catheter at the proximal portion of the Viabahn stent and with a 3-mm × 2-cm balloon catheter at the distal portion of the stent. After successful hemostasis of the femoral arteriotomy site with surgical repair, the 2 vascular sheaths were removed. Hemostasis of retrograde access site at the distal portion of the posterior tibial artery was performed with manual compression for 5 minutes. CT angiography taken 8 days after the procedure indicated markedly improved vascular flow through the Viabahn stent (Fig. 4).
Endovascular treatment has been adopted as an alternative to traditional fem-pop bypass surgery for superficial femoral artery occlusion. However, chronic occlusions are a serious technical challenge for endovascular interventional radiologists using conventional guide wires and catheters.
Failure to place the guidewire into the patent artery distal to the occlusion after intraluminal or subintimal passage, is the most frequent cause of unsuccessful recanalization (9). A reentry device such as the Outback catheter can mediate access to the true lumen and facilitate successful endovascular treatment of chronic total occlusions.
However, even with reentry devices, recanalization of CTOs is impossible when access cannot be gained to the patent true lumen. Previous studies have shown variable success rates of 50 to 100% with these re-entry devices in the treatment of infrainguinal CTO (278).
SAFARI technique is the other way to overcome failure of conventional endovascular techniques in these situations. It involves a retrograde wire approach from the distal puncture site, which usually facilitates wire passage through the occlusion site. However, this approach is not always successful in clinical practice, especially in patients with prior bypass graft, because of the scarring induced by previous operations (6). In this case, a wire inserted retrograde from the posterior tibial artery failed to cross the total occlusion site. During negotiation of the wire, the vessel was even perforated and the wire ended up in the extravascular space.
Previously, Bozlar et al. (6) reported an Outback catheter-assisted SAFARI technique, in which the Outback catheter was used in an antegrade fashion from the true lumen, in order to gain access to the subintimal space. They introduced the Outback catheter through an antegrade access point in the left limb of the aortobifemoral graft and targeted the retrograde advanced 4 Fr angiographic catheter that was located in the subintimal space. We used a target balloon, as compared to a 4 Fr catheter in the previous case as a target for the Outback catheter. Although they successfully entered the subintimal space upon the first pass, this would have been a challenge based on the small size of the subintimal space where the 4 Fr catheter was located. Expansion of a balloon in the target space, as in our case, facilitates easier targeting with the Outback catheter. It is also very easy to determine whether Outback needle puncture is accurate by monitoring perforation of the balloon. Furthermore, Bozlar's et al. technique was not possible in this case, since the retrograde advanced wire was not in the subintimal space, but instead in the extravascular space surrounding the scar tissue induced by the previous operation. This space was insufficient for angiographic catheter placement and the target space was attained only after balloon dilatation.
In this case, a Viabahn stent graft was used instead of a bare metal stent since the wire passage was achieved through the extravascular space and the occlusion was located in a portion that is easily bent with leg movement. Due to discrepancy of the luminal diameter between fem-pop bypass graft and Viabahn stent graft, Viabahn stent graft insertion was followed by ballooning with 3 mm diameter balloon for all length of the graft with a 4 mm balloon catheter for the proximal portion where the Viabahn stent graft was located in the lumen of fem-pop bypass graft.
Kirk et al. (10) previously reported cases of successful extraanatomical recanalization of occluded superficial femoral arteries using the Outback device. The vessel was perforated during subintimal angioplasty and they used the Outback device from the extravascular space to reach the distal true lumen and deployed a Viabahn stent graft. This technique is possible without a distal target for the Outback device, as in our case, because of the large patent distal femoral artery lumen. According to Kirk et al. (10), this procedure has some advantages. First, prolonged patency of the endograft is expected because of the outside location of calcification. Second, this procedure does not require open surgery. Extraanatomical stent graft deployment has great potential to bridge occluded vessels, even in cases where there is failure to achieve anatomical access (intra-luminal or subintimal) to the vessel, without surgical excision.
There are some limitations to the reported approach. This technique is considerably more complex and expensive than conventional angioplasty. Targeting the balloon catheter in the extravascular space with the Outback device has the potential risk of damage to other structures, such as adjacent veins and nerves.
In conclusion, when the antegrade-retrograde approach for managing CTO fails, placement of an extraanatomical stent graft using a balloon-targeted Outback reentry catheter might enable formation of a bridge across the occluded vessels, especially for patients with a history of bypass surgery.
Figures and Tables
Fig. 1
On initial diagnostic angiogram, the old and new fem-pop bypass grafts are not visualized due to complete occlusion with thrombosis. Popliteal artery and tibioperoneal trunk are also totally occluded. Anterior tibial artery is completely occluded at upper calf level containing some amount of thrombosis in the lumen (black arrow). The posterior tibial artery (white arrow) reconstituted through collateral branches supplies the foot.
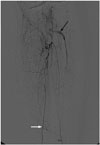
Fig. 2
Old fem-pop bypass graft (long black arrow) and new fem-pop bypass graft (long white arrow) are recanalized with Fogarty balloon and suction thrombectomy, respectively. However, multiple trials to pass the wire through occluded tibioperoneal trunk (short black arrow) failed. Some contrast material is noted in the extravascular space (short white arrow) through the perforation that was made during attempts at passing the wire through the occluded segment.
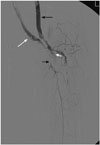
Fig. 3
Retrograde inserted 3 mm × 2 cm balloon catheter through posterior tibial artery access route at the ankle level (long black arrow) was targeted with Outback reentry catheter (long white arrow), which was inserted through recanalized old fem-pop bypass graft. Spillage of some contrast material from the balloon catheter into the adjacent soft tissue (short white arrows) right after accurate puncture of the balloon with Outback catheter, suggests that the balloon was located in the extravascular space instead of the subintimal space.
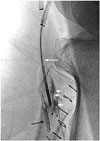
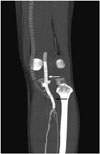
Fig. 4
On follow-up CT angiogram taken 8 days after procedure, good blood flow is visualized in the recanalized old fem-pop bypass graft (white arrow). Viabahn stent graft (black arrow) inserted through previous occluded segment is also highly patent, supplying good blood flow to the posterior tibial and peroneal arteries.
References
1. Bolia A, Miles KA, Brennan J, Bell PR. Percutaneous transluminal angioplasty of occlusions of the femoral and popliteal arteries by subintimal dissection. Cardiovasc Intervent Radiol. 1990; 13:357–363.
2. Jacobs DL, Motaganahalli RL, Cox DE, Wittgen CM, Peterson GJ. True lumen re-entry devices facilitate subintimal angioplasty and stenting of total chronic occlusions: initial report. J Vasc Surg. 2006; 43:1291–1296.
3. Green JS, Newland C, Fishwick G. Positive outcome following unsuccessful subintimal angioplasty. Eur J Vasc Endovasc Surg. 1998; 16:266–270.
4. Harthun NL, Cage DL, Spinosa DJ. Subintimal recanalization is safe and effective in treating chronic critical limb ischemia in selected patients. Am Surg. 2004; 70:479–482. discussion 482-483
5. Spinosa DJ, Harthun NL, Bissonette EA, Cage D, Leung DA, Angle JF, et al. Subintimal arterial flossing with antegrade-retrograde intervention (SAFARI) for subintimal recanalization to treat chronic critical limb ischemia. J Vasc Interv Radiol. 2005; 16:37–44.
6. Bozlar U, Shih MC, Harthun NL, Hagspiel KD. Outback catheter-assisted simultaneous antegrade and retrograde access for subintimal recanalization of peripheral arterial occlusion. Clin Imaging. 2008; 32:236–240.
7. Setacci C, Chisci E, de Donato G, Setacci F, Iacoponi F, Galzerano G. Subintimal angioplasty with the aid of a re-entry device for TASC C and D lesions of the SFA. Eur J Vasc Endovasc Surg. 2009; 38:76–87.
8. Wiesinger B, Steinkamp H, König C, Tepe G, Duda SH. Technical report and preliminary clinical data of a novel catheter for luminal re-entry after subintimal dissection. Invest Radiol. 2005; 40:725–728.
9. Bausback Y, Botsios S, Flux J, Werner M, Schuster J, Aithal J, et al. Outback catheter for femoropopliteal occlusions: immediate and long-term results. J Endovasc Ther. 2011; 18:13–21.
10. Kirk J, Wilson R, Kovacs F, Tennant W, Braithwaite B, Habib S. Successful extra-anatomical recanalization of occluded superficial femoral arteries using the Outback device--a report of 2 cases. Vasc Endovascular Surg. 2012; 46:62–65.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download