Abstract
Purpose
The aim of this study was to evaluate the diagnostic utility of the virtual touch tissue quantification (VTQ) technology for differentiating between benign and malignant thyroid nodules.
Materials and Methods
198 nodules (168 benign and 30 malignant nodules) identified in 164 patients with available VTQ velocity data and fine-needle aspiration cytology or post-surgical pathological results were included. The VTQ velocities of nodules and adjacent thyroid tissue were examined.
Results
Malignant nodules had a significantly higher VTQ velocity (3.06 ± 1.04 m/s, range: 1.90–6.46 m/s) than that of benign nodules (2.40 ± 0.85 m/s, range: 0.69–8.09 m/s) (p = 0.002). The VTQ velocity ratio between malignant nodules and adjacent thyroid tissue (1.39 ± 0.43, range: 0.89–2.65) was also statistically higher than that of benign nodules (1.15 ± 0.44, range: 0.26–3.47) (p = 0.008). The area under the receiver operating characteristic curve for the VTQ velocity was 0.72 with a cutoff point of 2.37 m/s and that of the VTQ velocity ratio was 0.68 with a cutoff point of 1.26. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for the VTQ velocity were 86.7%, 50.6%, 23.9%, 95.5%, and 56.1%, respectively and 60.0%, 72.0%, 27.7%, 91.0%, and 70.2%, respectively for the VTQ velocity ratio.
Ultrasonography is an accurate method for the detection of thyroid nodules, but it has limited utility in differentiating between benign and malignant thyroid nodules (1). Fine-needle aspiration (FNA) is a standard diagnostic procedure performed to obtain tissue for pathologic examination to determine whether a thyroid nodule is malignant. FNA is known to have a high specificity (60–98%) but varying sensitivity (54–90%) for the diagnosis of malignant thyroid nodules (2345). A classical criterion of malignancy is a nodule that is hard or firm upon palpation or with ultrasonography probe pressure (6). Therefore, assessment of tissue stiffness has become a viable method to differentiate thyroid nodules. Acoustic radiation force impulse (ARFI) imaging is an ultrasonographic elasticity imaging technology that noninvasively assesses tissue elasticity and quantifies the measured tissue stiffness as a velocity of ARFI. The quantitative application of ARFI technology is called virtual touch tissue quantification (VTQ) technology. The stiffer a tissue is, the greater the VTQ velocity is (7). VTQ has been applied to help distinguish between benign and malignant lesions in the breast, liver, and kidney (8910111213). More recently, studies have also been performed to investigate the application of VTQ technology to the thyroid (14151617); however, VTQ needs further testing before it can be utilized as a clinical imaging tool.
The aim of the present study was to evaluate the diagnostic utility of VTQ technology in differentiating between benign and malignant thyroid nodules.
This retrospective study was approved by the Institutional Review Board of our hospital, and the requirement for informed consent was waived. The study period was from September 2012 to August 2013. A total of 198 thyroid nodules in 164 consecutive patients (24 men and 140 women; mean age 53.8 ± 12.1 years, range 18–76 years) were retrospectively examined by one radiologist with 10 years of experience in thyroid ultrasonography and FNA. The flow chart for patient selection is shown in Fig. 1.
The final inclusion criteria were as follows: 1) nodules with a solid component that was 0.5 cm or greater in diameter; 2) sufficient adjacent thyroid tissue surrounding the nodule at the same depth in the region of interest (ROI); 3) nodules with an available VTQ velocity (0–10 m/s); and 4) nodules with a benign or malignant FNA cytology or post-surgical pathological results.
Nodules with Bethesda category I, III, IV, or V without postsurgical pathological results were excluded.
Conventional ultrasonography was done with an Acuson S2000 ultrasound system (Siemens Medical Solutions, Mountain View, CA, USA) equipped with a linear array transducer (bandwidth frequency of 4–9 MHz) and ARFI software. Patients were positioned in the supine position with a fully exposed neck. The morphologic characteristics, size, boundary, and echogenicity of the nodule were observed by conventional ultrasonography.
Switching to the ARFI mode, VTQ was utilized to measure the stiffness of the nodules and the adjacent normal thyroid tissues. The ROI measuring 0.5 × 0.6 cm was placed within the solid portion of the nodules. When a solid-and-cystic nodule was encountered, the ROI was centered within the solid component, and the FNA was also performed at the same location (Fig. 2). In cases of a large nodule with a heterogeneous solid portion, the ROI was placed in the most hypoechoic portion of the nodule. The VTQ velocity measurement was taken five times and completed within 3 minutes in most cases. The results were expressed in m/s with a measurement range of 0–10 m/s. The median value was selected as the VTQ velocity of the nodule. VTQ velocities greater than 10 m/s were not able to be evaluated by this ultrasound system and were displayed as 'x.xx m/s'. In cases without five valid measurements (numeric values), and more 'x.xx m/s' measurements than numeric values, the nodule was repeatedly measured to get an adequate result. VTQ measurements of adjacent normal thyroid tissue were also taken at the same depth and in the same manner as that of the nodules. In cases of heterogeneous thyroid parenchyma, such as thyroiditis, the VTQ velocity was measured in the same way as normal parenchymal tissue measurements. The VTQ velocity ratio between each nodule and the adjacent thyroid tissue was also calculated.
FNA was performed by the same radiologist who examined the conventional ultrasonography and ARFI imaging with a 21- or 25-gauge needle. The FNA result was reported according to the Bethesda system for reporting thyroid cytopathology (18). For a thyroid FNA specimen to be satisfactory for evaluation, at least 6 groups of benign follicular cells were required with each group composed of at least 10 cells. Non-diagnostic results were applied to specimens that were unsatisfactory due to obscuring blood, overly thick smears, air drying of alcohol-fixed smears, or an inadequate number of follicular cells. FNA cytology or post-surgical histopathological results were used as the standard of reference for the diagnosis of a benign or malignant thyroid nodule.
All measured data were expressed as the mean value ± standard deviation. A p value of less than 0.05 was considered as statistically significant. The VTQ velocities and VTQ velocity ratio between benign and malignant groups were compared by Student's t-test with SPSS 20 (SPSS Inc., Chicago, IL, USA). Receiver operating characteristic curve was used to assess the diagnostic performance of the VTQ velocity and VTQ velocity ratio. As a result, the best cutoff value was determined with MedCalc ver. 13.1.2.0 (MedCalc software, Mariakerke, Belgium).
In total, 168 benign nodules and 30 malignant nodules were included in this study. Demographic features of the patients and ultrasonographic features of the thyroid nodules are presented in Table 1. In 168 benign nodules, there were 166 benign follicular lesions and 2 cases of thyroiditis (1 Hashimoto thyroiditis and 1 lymphocytic thyroiditis). All 30 malignant nodules were papillary thyroid carcinoma.
Malignant nodules had significantly higher VTQ velocities (3.06 ± 1.04 m/s, range: 1.90–6.46 m/s) (Fig. 3) than those of benign nodules (2.40 ± 0.85 m/s, range: 0.69–8.09 m/s) (p = 0.002). The optimal cutoff velocity was 2.37 m/s for accurate differentiation between benign and malignant thyroid nodules. The area under the receiver operating characteristic curve was 0.72. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 86.7%, 50.6%, 23.9%, 95.5%, and 56.1%, respectively.
The VTQ velocity ratios between malignant nodules and adjacent thyroid tissues were also statistically higher than those of benign nodules (Table 2). The area under the receiver operating characteristic curve was 0.68 with an optimal cutoff point of 1.26. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 60.0%, 72.0%, 27.7%, 91.0%, and 70.2%, respectively. The mean values and distributions of the VTQ velocities and VTQ velocity ratio in benign and malignant groups are presented in Table 3. In the present study, only 4 of the 30 malignant nodules had VTQ velocities lower than the cutoff point (2.37 m/s) (Fig. 4).
VTQ velocities of adjacent thyroid parenchyma demonstrated no significant difference between the malignant (2.26 ± 0.49 m/s, range: 1.38–3.10 m/s) and benign groups (2.18 ± 0.52 m/s, range: 1.07–3.42 m/s) (p = 0.444).
Real-time elastography has been introduced into clinical practice and has enabled the determination of tissue elasticity. The calculation of the tissue elasticity distribution is performed in real-time and the examination results are displayed as color-coded images over conventional B-mode images. However, real-time elastography has been challenged and criticized for its operator dependency and lack of quantitative information. Thus, its use is limited in clinical practice (19).
ARFI imaging is an ultrasonographic elasticity imaging technology that allows for quantification of tissue stiffness. The quantitative application of ARFI technology, or VTQ technology, does not depend on the examination skills of the operator when vibrating or stressing the tissue. Therefore, VTQ is able to provide information on the tissue elasticity in an objective manner (20). Grazhdani et al. (14) obtained good inter-observer agreement with VTQ when differentiating thyroid nodules. VTQ has also been applied to help distinguish between benign and malignant lesions in the breast, liver and kidney (8910111213).
In the present study, the VTQ velocities of malignant nodules were 3.06 ± 1.04 m/s. This finding is slightly lower than that of previous studies by Friedrich-Rust et al. (15) (3.73 ± 1.16 m/s) and Gu et al. (16) (3.94 ± 1.39 m/s). Differences in ROI depth, ROI size, and size of the enrolled nodules may affect the VTQ velocity results. Recently, Jung et al. (21) reported an accuracy of 86% with a cutoff value of 3.28 m/s. However, they concluded that the combined evaluation of ARFI VTQ value alongside conventional B-mode ultrasonography may have better diagnostic performance than VTQ value evaluation alone.
In the present study, only 4 of 30 malignant nodules were found to have VTQ velocities lower than the cutoff point (2.37 m/s) (Fig. 4). Among these 4 malignant nodules, 1 nodule was confirmed by histopathologic examination as papillary carcinoma in the background of follicular adenoma. Because the size and shape of the ROI of the VTQ velocity measurement is fixed, some adjacent thyroid parenchyma could be included in the ROI, which could affect the result. In addition, there cancer cell degeneration may also take place, which could soften the malignant tissue.
The VTQ velocity ratio represents the relative hardness of nodules. In our study, the diagnostic performance of the VTQ velocity ratio was lower than the VTQ velocity. This result was consistent with that of previous studies (17). When the thyroid has diffuse disease, changes in the composition of adjacent thyroid tissue may be present (22). Therefore, the VTQ velocity ratio may not represent the true consistency of the nodules.
FNA is the most accurate and cost-effective preoperative diagnostic method for evaluating thyroid nodules. Although FNA is a minimally invasive and highly accurate procedure, its major drawback is that 5–10% of procedures yield non-diagnostic results (23). As for repeated non-diagnostic cytology, diagnostic surgical excision could be considered based on the clinical and ultrasonographic features (6).
To reduce unnecessary diagnostic surgery, a reliable and non-invasive method to differentiate malignant from benign thyroid nodules is needed beyond conventional ultrasonography. In our study, among the 5 benign nodules with histopathological results after surgery, a 0.8 cm sized hypoehoic solid nodule with isoechoic foci on the conventional ultrasonography and a lower VTQ velocity than the cutoff value was identified (Fig. 5). Because a few degenerated atypical cells and amorphous materials were identified in the aspirated specimen, re-aspiration was recommended by the pathologist. After repeated FNA yielded similar results, a total thyroidectomy was performed. However, the final pathologic result was nodular hyperplasia. Moon et al. (23) divided thyroid nodules into three categories: suspicious malignant nodules, probably benign nodules, and indeterminate nodules. Indeterminate nodules include nodules having ultrasonographic findings with neither malignant (taller-than wide shape, spiculated margin, marked hypoechogenicity, microcalcifications, and macrocalcifications) nor benign features (simple cyst, predominantly cystic or predominantly cystic nodule with reverberating artifacts and spongiform nodules). In cases of indeterminate nodules with non-diagnostic FNA results, such as our case, VTQ may be helpful in the differentiation of malignant from benign thyroid nodules.
In the present study, we utilized a much smaller ROI (5 × 6 mm) than that of a previous study by Grazhdani et al. (14). As a result, thyroid nodules smaller than 10 mm in diameter were included in our study.
Conventional ultrasonography, VTQ velocity measurement, and FNA were performed by only one radiologist at the same time, with the intent of minimizing inter-observer variability and obtain more reliable cytological results by matching the ROI and FNA site.
There were several limitations in our study. Firstly, the reference standard was FNA cytology, and only 25 nodules were confirmed by postsurgical histopathology. To improve the accuracy of the FNA result as a reference standard, we included thyroid nodules with the diagnostic category "benign" or "malignant". We excluded nodules with the diagnostic categories "non-diagnostic", "atypia" or "suspicious for malignancy" with no surgical confirmation during the study period. Secondly, only a few histopathological subtypes of thyroid lesions were included (30 papillary thyroid carcinomas). Further studies with a greater variety of malignant and benign lesions in a larger sample size are needed to establish the clinical value of VTQ in the differential diagnosis of thyroid nodules. Thirdly, VTQ results may be inaccurate due to ROI placement in some nodules. In cases of thyroid nodules with an irregular shape or with non-symmetric dimensions, adjacent thyroid parenchyma may have been inadvertently included in the ROI and could thereby affect the result. In cases of large nodules with a heterogeneous solid component, selection bias of the VTQ velocity may have resulted from ROI placement. Fourthly, VTQ velocities over 10 m/s were not measured properly and displayed as 'x.xx m/s' in the ultrasonography system. This made the calculation of the mean VTQ velocity impossible in some cases. Therefore, the median value was selected as the VTQ velocity of the nodules in this study. Technical optimization to increase the range of velocity detection is needed to provide proper information on the stiffness of such nodules. Finally, investigating the VTQ velocity values combined with conventional ultrasonography was not performed in our study.
In conclusion, VTQ provides quantitative information on tissue hardness and consistency with a high negative predictive value, which may be helpful in differentiating malignant from benign thyroid nodules. Further studies are needed to establish the diagnostic role of VTQ in clinical practice.
Figures and Tables
 | Fig. 1The flow chart of the selection of the patients with thyroid nodules. Nn = number of nodules, Np = number of patients, VTQ = virtual touch tissue quantification |
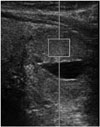 | Fig. 2VTQ velocity measurement and FNA of a 2.6 cm sized mixed solid-and-cystic thyroid nodule in a 70-year-old male. ROI is placed in the center of the solid portion of the nodule.FNA = fine-needle aspiration, ROI = region of interest, VTQ = virtual touch tissue quantification
|
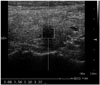 | Fig. 3The median value of VTQ velocities of a 0.6 sized hypoechoic nodule that is taller than it is wide is 3.30 m/s (> 2.37 m/s: cutoff point) in a 60-year-old female with papillary thyroid carcinoma.VTQ = virtual touch tissue quantification
|
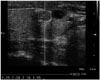 | Fig. 4The median VTQ velocities of a 0.8 cm sized isoechoic nodule is 2.15 m/s (< 2.37 m/s: cutoff point) in a 36-year-old male with papillary thyroid carcinoma.VTQ = virtual touch tissue quantification
|
 | Fig. 5Nodular hyperplasia confirmed by post-surgical histopathology in a 58-year-old male.
A. Conventional ultrasonography shows a 0.8 cm sized hypoechoic solid nodule with echogenic foci in the left thyroid gland.
B. The median value of VTQ velocities of the nodule is 1.55 m/s (< 2.37 m/s: cutoff point).
VTQ = virtual touch tissue quantification
|
Table 1
The Characteristics of Patients and Ultrasonographic Features of the Thyroid Nodules

Table 2
VTQ Velocity and VTQ Velocity Ratio of Benign and Malignant Nodules

References
1. Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, et al. Management of thyroid nodules detected at US: society of radiologists in ultrasound consensus conference statement. Radiology. 2005; 237:794–800.
2. Tee YY, Lowe AJ, Brand CA, Judson RT. Fine-needle aspiration may miss a third of all malignancy in palpable thyroid nodules: a comprehensive literature review. Ann Surg. 2007; 246:714–720.
3. Peng Y, Wang HH. A meta-analysis of comparing fineneedle aspiration and frozen section for evaluating thyroid nodules. Diagn Cytopathol. 2008; 36:916–920.
4. Oertel YC, Miyahara-Felipe L, Mendoza MG, Yu K. Value of repeated fine needle aspirations of the thyroid: an analysis of over ten thousand FNAs. Thyroid. 2007; 17:1061–1066.
5. La Rosa GL, Belfiore A, Giuffrida D, Sicurella C, Ippolito O, Russo G, et al. Evaluation of the fine needle aspiration biopsy in the preoperative selection of cold thyroid nodules. Cancer. 1991; 67:2137–2141.
6. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006; 16:109–142.
7. Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009; 252:595–604.
8. Tozaki M, Isobe S, Fukuma E. Preliminary study of ultrasonographic tissue quantification of the breast using the acoustic radiation force impulse (ARFI) technology. Eur J Radiol. 2011; 80:e182–e187.
9. Meng W, Zhang G, Wu C, Wu G, Song Y, Lu Z. Preliminary results of acoustic radiation force impulse (ARFI) ultrasound imaging of breast lesions. Ultrasound Med Biol. 2011; 37:1436–1443.
10. Shuang-Ming T, Ping Z, Ying Q, Li-Rong C, Ping Z, Rui-Zhen L. Usefulness of acoustic radiation force impulse imaging in the differential diagnosis of benign and malignant liver lesions. Acad Radiol. 2011; 18:810–815.
11. Cho SH, Lee JY, Han JK, Choi BI. Acoustic radiation force impulse elastography for the evaluation of focal solid hepatic lesions: preliminary findings. Ultrasound Med Biol. 2010; 36:202–208.
12. Yoneda M, Suzuki K, Kato S, Fujita K, Nozaki Y, Hosono K, et al. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010; 256:640–647.
13. Clevert DA, Stock K, Klein B, Slotta-Huspenina J, Prantl L, Heemann U, et al. Evaluation of acoustic radiation force impulse (ARFI) imaging and contrast-enhanced ultrasound in renal tumors of unknown etiology in comparison to histological findings. Clin Hemorheol Microcirc. 2009; 43:95–107.
14. Grazhdani H, Cantisani V, Lodise P, Di Rocco G, Proietto MC, Fioravanti E, et al. Prospective evaluation of acoustic radiation force impulse technology in the differentiation of thyroid nodules: accuracy and interobserver variability assessment. J Ultrasound. 2014; 17:13–20.
15. Friedrich-Rust M, Romenski O, Meyer G, Dauth N, Holzer K, Grünwald F, et al. Acoustic radiation force impulse-imaging for the evaluation of the thyroid gland: a limited patient feasibility study. Ultrasonics. 2012; 52:69–74.
16. Gu J, Du L, Bai M, Chen H, Jia X, Zhao J, et al. Preliminary study on the diagnostic value of acoustic radiation force impulse technology for differentiating between benign and malignant thyroid nodules. J Ultrasound Med. 2012; 31:763–771.
17. Zhang FJ, Han RL. The value of acoustic radiation force impulse (ARFI) in the differential diagnosis of thyroid nodules. Eur J Radiol. 2013; 82:e686–e690.
18. Cibas ES, Ali SZ. The bethesda system for reporting thyroid cytopathology. Am J Clin Pathol. 2009; 132:658–665.
19. Lippolis PV, Tognini S, Materazzi G, Polini A, Mancini R, Ambrosini CE, et al. Is elastography actually useful in the presurgical selection of thyroid nodules with indeterminate cytology? J Clin Endocrinol Metab. 2011; 96:E1826–E1830.
20. Nightingale K, McAleavey S, Trahey G. Shear-wave generation using acoustic radiation force: in vivo and ex vivo results. Ultrasound Med Biol. 2003; 29:1715–1723.
21. Jung WS, An YY, Ihn YK, Park YH. The diagnostic performance of acoustic radiation force impulse elasticity imaging to differentiate malignant from benign thyroid nodules: comparison with conventional B-mode sonographic findings. J Korean Soc Radiol. 2016; 74:96–104.
22. Sporea I, Vlad M, Bota S, Sirli RL, Popescu A, Danila M, et al. Thyroid stiffness assessment by acoustic radiation force impulse elastography (ARFI). Ultraschall Med. 2011; 32:281–285.
23. Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011; 12:1–14.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download